Molecular diversity of Giardia duodenalis in children under 5 years from the Manhiça district, Southern Mozambique enrolled in a matched case-control study on the aetiology of diarrhoea
- PMID: 33465074
- PMCID: PMC7846004
- DOI: 10.1371/journal.pntd.0008987
Molecular diversity of Giardia duodenalis in children under 5 years from the Manhiça district, Southern Mozambique enrolled in a matched case-control study on the aetiology of diarrhoea
Abstract
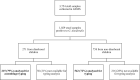
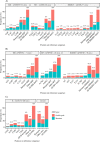
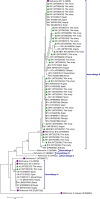
Giardia duodenalis is an enteric parasite commonly detected in children. Exposure to this organism may lead to asymptomatic or symptomatic infection. Additionally, early-life infections by this protozoan have been associated with impaired growth and cognitive function in poor resource settings. The Global Enteric Multicenter Study (GEMS) in Mozambique demonstrated that G. duodenalis was more frequent among controls than in diarrhoeal cases (≥3 loosing stools in the previous 24 hours). However, no molecular investigation was conducted to ascertain the molecular variability of the parasite. Therefore, we describe here the frequency and genetic diversity of G. duodenalis infections in children younger than five years of age with and without diarrhoea from the Manhiça district in southern Mozambique enrolled in the context of GEMS. Genomic DNA from 757 G. duodenalis-positive stool samples by immunoassay collected between 2007-2012, were reanalysed by multiplex PCR targeting the E1-HP and C1-P21 genes for the differentiation of assemblages A and B. Overall, 47% (353) of the samples were successfully amplified in at least one locus. Assemblage B accounted for 90% (319/353) of all positives, followed by assemblage A (8%, 29/353) and mixed A+B infections (1%, 5/353). No association between the presence of a given assemblage and the occurrence of diarrhoea could be demonstrated. A total of 351 samples were further analysed by a multi-locus sequence genotyping (MLSG) approach at the glutamate dehydrogenase (gdh), ß-giardin (bg) and triose phosphate isomerase (tpi) genes. Overall, 63% (222/351) of samples were genotyped and/or sub-genotyped in at least one of the three markers. Sequence analysis revealed the presence of assemblages A (10%; 23/222) and B (90%; 199/222) with high molecular diversity at the nucleotide level within the latter; no mixed infections were identified under the MLSG scheme. Assemblage A sequences were assigned to sub-assemblages AI (0.5%, 1/222), AII (7%, 15/222) or ambiguous AII/AIII (3%, 7/222). Within assemblage B, sequences were assigned to sub-assemblages BIII (13%, 28/222), BIV (14%, 31/222) and ambiguous BIII/BIV (59%, 132/222). BIII/BIV sequences accumulated the majority of the single nucleotide polymorphisms detected, particularly in the form of double peaks at chromatogram inspection. This study demonstrated that the occurrence of gastrointestinal illness (diarrhoea) was not associated to a given genotype of G. duodenalis in Mozambican children younger than five years of age. The assemblage B of the parasite was responsible for nine out of ten infections detected in this paediatric population. The extremely high genetic diversity observed within assemblage B isolates was compatible with an hyperendemic epidemiological scenario where infections and reinfections were common. The obtained molecular data may be indicative of high coinfection rates by different G. duodenalis assemblages/sub-assemblages and/or genetic recombination events, although the exact contribution of both mechanisms to the genetic diversity of the parasite remains unknown.
Conflict of interest statement
The authors have no competing interests.
Figures
References
-
- Wang H, Naghavi M, Allen C, Barber RM, Carter A, Casey DC, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388: 1459–1544. 10.1016/S0140-6736(16)31012-1 - DOI - PMC - PubMed
-
- Wang H, Coates MM, Coggeshall M, Dandona L, Fraser M, Fullman N, et al. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388: 1725–1774. 10.1016/S0140-6736(16)31575-6 - DOI - PMC - PubMed
-
- Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Reiner RC, et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17: 909–948. 10.1016/S1473-3099(17)30276-1 - DOI - PMC - PubMed
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet. 2013;382: 209–222. 10.1016/S0140-6736(13)60844-2 - DOI - PubMed
-
- Kotloff KL, Nasrin D, Blackwelder WC, Wu Y, Farag T, Panchalingham S, et al. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: a 12-month case-control study as a follow-on to the Global Enteric Multicenter St. Lancet Glob Heal. 2019;7: e568–e584. 10.1016/S2214-109X(19)30076-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

