Blocking IL-10 signaling with soluble IL-10 receptor restores in vitro specific lymphoproliferative response in dogs with leishmaniasis caused by Leishmania infantum
- PMID: 33465107
- PMCID: PMC7815104
- DOI: 10.1371/journal.pone.0239171
Blocking IL-10 signaling with soluble IL-10 receptor restores in vitro specific lymphoproliferative response in dogs with leishmaniasis caused by Leishmania infantum
Abstract
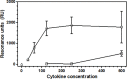
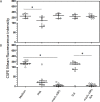
rIL-10 plays a major role in restricting exaggerated inflammatory and immune responses, thus preventing tissue damage. However, the restriction of inflammatory and immune responses by IL-10 can also favor the development and/or persistence of chronic infections or neoplasms. Dogs that succumb to canine leishmaniasis (CanL) caused by L. infantum develop exhaustion of T lymphocytes and are unable to mount appropriate cellular immune responses to control the infection. These animals fail to mount specific lymphoproliferative responses and produce interferon gamma and TNF-alpha that would activate macrophages and promote destruction of intracellular parasites. Blocking IL-10 signaling may contribute to the treatment of CanL. In order to obtain a tool for this blockage, the present work endeavored to identify the canine casIL-10R1 amino acid sequence, generate a recombinant baculovirus chromosome encoding this molecule, which was expressed in insect cells and subsequently purified to obtain rcasIL-10R1. In addition, rcasIL-10R1 was able to bind to homologous IL-10 and block IL-10 signaling pathway, as well as to promote lymphoproliferation in dogs with leishmaniasis caused by L. infantum.
Conflict of interest statement
No authors have competing interests.
Figures


Similar articles
-
Combined in vitro IL-12 and IL-15 stimulation promotes cellular immune response in dogs with visceral leishmaniasis.PLoS Negl Trop Dis. 2020 Jan 21;14(1):e0008021. doi: 10.1371/journal.pntd.0008021. eCollection 2020 Jan. PLoS Negl Trop Dis. 2020. PMID: 31961868 Free PMC article.
-
IL-10 receptor blockade controls the in vitro infectivity of Leishmania infantum and promotes a Th1 activation in PBMC of dogs with visceral leishmaniasis.Mol Immunol. 2021 Sep;137:20-27. doi: 10.1016/j.molimm.2021.06.014. Epub 2021 Jun 26. Mol Immunol. 2021. PMID: 34182228
-
Interleukin-12 augments a Th1-type immune response manifested as lymphocyte proliferation and interferon gamma production in Leishmania infantum-infected dogs.Int J Parasitol. 2005 Jan;35(1):63-73. doi: 10.1016/j.ijpara.2004.10.015. Epub 2004 Dec 8. Int J Parasitol. 2005. PMID: 15619517
-
Vaccination using live attenuated Leishmania donovani centrin deleted parasites induces protection in dogs against Leishmania infantum.Vaccine. 2015 Jan 3;33(2):280-8. doi: 10.1016/j.vaccine.2014.11.039. Epub 2014 Dec 1. Vaccine. 2015. PMID: 25475955
-
Cutaneous immune mechanisms in canine leishmaniosis due to Leishmania infantum.Vet Immunol Immunopathol. 2015 Feb 15;163(3-4):94-102. doi: 10.1016/j.vetimm.2014.11.011. Epub 2014 Nov 20. Vet Immunol Immunopathol. 2015. PMID: 25555497 Review.
Cited by
-
Dual blockade of IL-10 and PD-1 leads to control of SIV viral rebound following analytical treatment interruption.Nat Immunol. 2024 Oct;25(10):1900-1912. doi: 10.1038/s41590-024-01952-4. Epub 2024 Sep 12. Nat Immunol. 2024. PMID: 39266691 Free PMC article.
-
Effects of Doxycycline Treatment on Hematological Parameters, Viscosity, and Cytokines in Canine Monocytic Ehrlichiosis.Biology (Basel). 2023 Aug 16;12(8):1137. doi: 10.3390/biology12081137. Biology (Basel). 2023. PMID: 37627021 Free PMC article.
-
Is the Prevalence of Leishmania infantum Linked to Breeds in Dogs? Characterization of Seropositive Dogs in Ibiza.Animals (Basel). 2021 Sep 2;11(9):2579. doi: 10.3390/ani11092579. Animals (Basel). 2021. PMID: 34573545 Free PMC article.
-
Nasal Immunization With Small Molecule Mast Cell Activators Enhance Immunity to Co-Administered Subunit Immunogens.Front Immunol. 2021 Sep 10;12:730346. doi: 10.3389/fimmu.2021.730346. eCollection 2021. Front Immunol. 2021. PMID: 34566991 Free PMC article.
References
-
- Abbas AK, Lichtman AH, Pillai S, Baker DL, Baker A: Immune receptors and signal transduction In: Cellular and molecular immunology. 9th edn. Philadelphia: Elsevier; 2018.
-
- Cavaillon JM: Pro- versus anti-inflammatory cytokines: myth or reality. Cell Mol Biol (Noisy-le-grand) 2001, 47(4):695–702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

