Efficacy of canakinumab in mild or severe COVID-19 pneumonia
- PMID: 33465283
- PMCID: PMC8013503
- DOI: 10.1002/iid3.400
Efficacy of canakinumab in mild or severe COVID-19 pneumonia
Abstract
Background: Clinicians all around the world are currently experiencing a pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Several therapeutic strategies have been used until now but, to date, there is no specific therapy to treat SARS-CoV-2 infection. In this study, we used canakinumab, a human monoclonal antibody targeting interleukin-1 beta to improve respiratory function and laboratory parameters compared with standard therapy (hydroxycloroquine plus lopinavir/ritonavir).
Methods: We enrolled 34 patients with mild or severe non intensive care unit (ICU) coronavirus disease 2019 (COVID-19): 17 patients treated with standard therapy and 17 patients treated with a subcutaneous single dose of canakinumab 300 mg. We collected data about oxygen supports and laboratory parameters such as inflammation indices and hemogasanalysis. We compared the data collected before the administration of canakinumab (T0), 3 days after T0 (T1) and 7 days after T0 (T2) with the same data from patients taking the standard therapy.
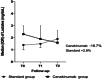
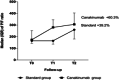
Results: We observed a reduction in inflammation indices and a significant and rapid increase in P/F ratio in canakinumab group, with improvement of 60.3% after the administration. We reported a significant reduction in oxygen flow in patients treated with canakinumab (-28.6% at T1 vs. T0 and -40.0% at T2 vs. T1). Conversely, the standard group increased the supply of high oxygen at T1 versus T0 (+66.7%), but reduced oxygen flows at T2 versus T1 (-40.0%).
Conclusion: In hospitalized adult patients with mild or severe non ICU COVID-19, canakinumab could be a valid therapeutic option. Canakinumab therapy causes rapid and long-lasting improvement in oxygenation levels in the absence of any severe adverse events.
Keywords: IL1-β; SARS COV2; monoclonal antibody; safety; therapy.
© 2020 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures
References
-
- Balachandar V, Mahalaxmi I, Kaavya J, et al. COVID‐19: emerging protective measures. Eur Rev Med Pharmacol Sci. 2020;24(6):3422‐3425. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

