Review: Neural Mechanisms of Tinnitus and Hyperacusis in Acute Drug-Induced Ototoxicity
- PMID: 33465315
- PMCID: PMC9126116
- DOI: 10.1044/2020_AJA-20-00023
Review: Neural Mechanisms of Tinnitus and Hyperacusis in Acute Drug-Induced Ototoxicity
Abstract
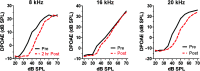
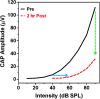
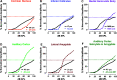
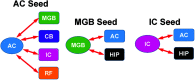
Purpose Tinnitus and hyperacusis are debilitating conditions often associated with age-, noise-, and drug-induced hearing loss. Because of their subjective nature, the neural mechanisms that give rise to tinnitus and hyperacusis are poorly understood. Over the past few decades, considerable progress has been made in deciphering the biological bases for these disorders using animal models. Method Important advances in understanding the biological bases of tinnitus and hyperacusis have come from studies in which tinnitus and hyperacusis are consistently induced with a high dose of salicylate, the active ingredient in aspirin. Results Salicylate induced a transient hearing loss characterized by a reduction in otoacoustic emissions, a moderate cochlear threshold shift, and a large reduction in the neural output of the cochlea. As the weak cochlear neural signals were relayed up the auditory pathway, they were progressively amplified so that the suprathreshold neural responses in the auditory cortex were much larger than normal. Excessive central gain (neural amplification), presumably resulting from diminished inhibition, is believed to contribute to hyperacusis and tinnitus. Salicylate also increased corticosterone stress hormone levels. Functional imaging studies indicated that salicylate increased spontaneous activity and enhanced functional connectivity between structures in the central auditory pathway and regions of the brain associated with arousal (reticular formation), emotion (amygdala), memory/spatial navigation (hippocampus), motor planning (cerebellum), and motor control (caudate/putamen). Conclusion These results suggest that tinnitus and hyperacusis arise from aberrant neural signaling in a complex neural network that includes both auditory and nonauditory structures.
Figures



Similar articles
-
Functional Neuroanatomy of Salicylate- and Noise-Induced Tinnitus and Hyperacusis.Curr Top Behav Neurosci. 2021;51:133-160. doi: 10.1007/7854_2020_156. Curr Top Behav Neurosci. 2021. PMID: 32653998
-
Tinnitus and hyperacusis: Contributions of paraflocculus, reticular formation and stress.Hear Res. 2017 Jun;349:208-222. doi: 10.1016/j.heares.2017.03.005. Epub 2017 Mar 7. Hear Res. 2017. PMID: 28286099 Free PMC article.
-
Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network.Elife. 2015 May 12;4:e06576. doi: 10.7554/eLife.06576. Elife. 2015. PMID: 25962854 Free PMC article.
-
Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis.Prog Neurobiol. 2013 Dec;111:17-33. doi: 10.1016/j.pneurobio.2013.08.002. Epub 2013 Sep 6. Prog Neurobiol. 2013. PMID: 24012803 Review.
-
Auditory sensori-neural alterations induced by salicylate.Prog Neurobiol. 2000 Dec;62(6):583-631. doi: 10.1016/s0301-0082(00)00027-7. Prog Neurobiol. 2000. PMID: 10880852 Review.
Cited by
-
Functional Connectivity Alterations and Molecular Characterization of the Anterior Cingulate Cortex in Tinnitus Pathology without Hearing Loss.Adv Sci (Weinh). 2024 Jan;11(3):e2304709. doi: 10.1002/advs.202304709. Epub 2023 Nov 27. Adv Sci (Weinh). 2024. PMID: 38009798 Free PMC article.
-
Thalamo-cortical neural mechanism of sodium salicylate-induced hyperacusis and anxiety-like behaviors.Commun Biol. 2024 Oct 18;7(1):1346. doi: 10.1038/s42003-024-07040-5. Commun Biol. 2024. PMID: 39420035 Free PMC article.
-
The Medial Olivocochlear Efferent Pathway Potentiates Cochlear Amplification in Response to Hearing Loss.J Neurosci. 2025 Apr 9;45(15):e2103242025. doi: 10.1523/JNEUROSCI.2103-24.2025. J Neurosci. 2025. PMID: 39984203
-
Implications of Transcranial Magnetic Stimulation as a Treatment Modality for Tinnitus.J Clin Med. 2021 Nov 20;10(22):5422. doi: 10.3390/jcm10225422. J Clin Med. 2021. PMID: 34830704 Free PMC article. Review.
-
Identifying tinnitus in mice by tracking the motion of body markers in response to an acoustic startle.Front Neurosci. 2024 Aug 7;18:1452450. doi: 10.3389/fnins.2024.1452450. eCollection 2024. Front Neurosci. 2024. PMID: 39170684 Free PMC article.
References
-
- Alpini, D. , & Cesarani, A. (2006). Tinnitus as an alarm bell: Stress reaction tinnitus model. ORL, 68(1), 31–37. https://doi.org/10.1159/000090488 - PubMed
-
- Anders, S. , Eippert, F. , Weiskopf, N. , & Veit, R. (2008). The human amygdala is sensitive to the valence of pictures and sounds irrespective of arousal: An fMRI study. Social Cognitive and Affective Neuroscience, 3(3), 233–243. https://doi.org/10.1093/scan/nsn017 - PMC - PubMed
-
- Auerbach, B. D. , Rodrigues, P. V. , & Salvi, R. J. (2014). Central gain control in tinnitus and hyperacusis. Frontiers in Neurology, 5, 206. https://doi.org/10.3389/fneur.2014.00206 - PMC - PubMed
-
- Avery, M. A. , Sheehan, A. E. , Kerr, K. S. , Wang, J. , & Freeman, M. R. (2009). WldS requires Nmnat1 enzymatic activity and N16–VCP interactions to suppress Wallerian degeneration. Journal of Cell Biology, 184(4), 501–513. https://doi.org/10.1083/jcb.200808042 - PMC - PubMed
-
- Azizi, S. A. , Burne, R. A. , & Woodward, D. J. (1985). The auditory corticopontocerebellar projection in the rat: Inputs to the paraflocculus and midvermis. An anatomical and physiological study. Experimental Brain Research, 59(1), 36–49. https://doi.org/10.1007/BF00237663 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

