Dissecting the low catalytic capability of flavin-dependent halogenases
- PMID: 33465708
- PMCID: PMC7948982
- DOI: 10.1074/jbc.RA120.016004
Dissecting the low catalytic capability of flavin-dependent halogenases
Abstract
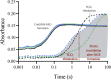
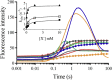
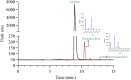
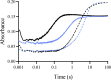
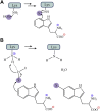
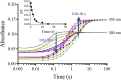
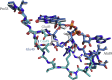
Although flavin-dependent halogenases (FDHs) are attractive biocatalysts, their practical applications are limited because of their low catalytic efficiency. Here, we investigated the reaction mechanisms and structures of tryptophan 6-halogenase (Thal) from Streptomyces albogriseolus using stopped-flow, rapid-quench flow, quantum/mechanics molecular mechanics calculations, crystallography, and detection of intermediate (hypohalous acid [HOX]) liberation. We found that the key flavin intermediate, C4a-hydroperoxyflavin (C4aOOH-FAD), formed by Thal and other FDHs (tryptophan 7-halogenase [PrnA] and tryptophan 5-halogenase [PyrH]), can react with I-, Br-, and Cl- but not F- to form C4a-hydroxyflavin and HOX. Our experiments revealed that I- reacts with C4aOOH-FAD the fastest with the lowest energy barrier and have shown for the first time that a significant amount of the HOX formed leaks out as free HOX. This leakage is probably a major cause of low product coupling ratios in all FDHs. Site-saturation mutagenesis of Lys79 showed that changing Lys79 to any other amino acid resulted in an inactive enzyme. However, the levels of liberated HOX of these variants are all similar, implying that Lys79 probably does not form a chloramine or bromamine intermediate as previously proposed. Computational calculations revealed that Lys79 has an abnormally lower pKa compared with other Lys residues, implying that the catalytic Lys may act as a proton donor in catalysis. Analysis of new X-ray structures of Thal also explains why premixing of FDHs with reduced flavin adenine dinucleotide generally results in abolishment of C4aOOH-FAD formation. These findings reveal the hidden factors restricting FDHs capability which should be useful for future development of FDHs applications.
Keywords: QM/MM calculations; X-ray structures; flavin monooxygenase; halogenase; kinetics; stopped-flow.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflict of interest with the contents of this article.
Figures




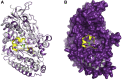
References
-
- Jeschke P. The unique role of halogen substituents in the design of modern agrochemicals. Pest Manag. Sci. 2009;66:10–27. - PubMed
-
- Lu Y., Liu Y., Xu Z., Li H., Liu H., Zhu W. Halogen bonding for rational drug design and new drug discovery. Expert Opin. Drug Discov. 2012;7:375–383. - PubMed
-
- Lu Y., Shi T., Wang Y., Yang H., Yan X., Luo X., Jiang H., Zhu W. Halogen bonding-a novel interaction for rational drug design? J. Med. Chem. 2009;52:2854–2862. - PubMed
-
- Marcelo Zaldini H., Suellen Melo T.C., Diogo Rodrigo M.M., Walter Filgueira de Azevedo J., Ana Cristina Lima L. Halogen atoms in the modern medicinal chemistry: hints for the drug design. Curr. Drug Targets. 2010;11:303–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

