Neuroprotective Effects of Extracts from Tiger Milk Mushroom Lignosus rhinocerus Against Glutamate-Induced Toxicity in HT22 Hippocampal Neuronal Cells and Neurodegenerative Diseases in Caenorhabditis elegans
- PMID: 33466350
- PMCID: PMC7824744
- DOI: 10.3390/biology10010030
Neuroprotective Effects of Extracts from Tiger Milk Mushroom Lignosus rhinocerus Against Glutamate-Induced Toxicity in HT22 Hippocampal Neuronal Cells and Neurodegenerative Diseases in Caenorhabditis elegans
Abstract
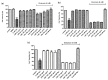
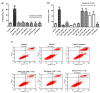
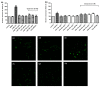
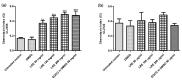
Despite the Tiger Milk Mushroom Lignosus rhinocerus (LR) having been used as a traditional medicine, little is known about the neuroprotective effects of LR extracts. This study aims to investigate the neuroprotective effect of three extracts of LR against glutamate-induced oxidative stress in mouse hippocampal (HT22) cells as well as to determine their effect in Caenorhabditis elegans. In vitro, we assessed the toxicity of three LR extracts (ethanol extract (LRE), cold-water extract (LRC) and hot-water extract (LRH)) and their protective activity by MTT assay, Annexin V-FITC/propidium iodide staining, Mitochondrial Membrane Potential (MMP) and intracellular ROS accumulation. Furthermore, we determined the expression of antioxidant genes (catalase (CAT), superoxide dismutase (SOD1 and SOD2) and glutathione peroxidase (GPx)) by qRT-PCR. In vivo, we investigated the neuroprotective effect of LRE, not only against an Aβ-induced deficit in chemotaxis behavior (Alzheimer model) but also against PolyQ40 formation (model for Morbus Huntington) in transgenic C. elegans. Only LRE significantly reduced both apoptosis and intracellular ROS levels and significantly increased the expression of antioxidant genes after glutamate-induced oxidative stress in HT22 cells. In addition, LRE significantly improved the Chemotaxis Index (CI) in C. elegans and significantly decreased PolyQ40 aggregation. Altogether, the LRE exhibited neuroprotective properties both in vitro and in vivo.
Keywords: Caenorhabditis elegans; HT22; Lignosus rhinocerus; PolyQ40; chemotaxis; glutamate toxicity; neuroprotection; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

