FUT8 Alpha-(1,6)-Fucosyltransferase in Cancer
- PMID: 33466384
- PMCID: PMC7795606
- DOI: 10.3390/ijms22010455
FUT8 Alpha-(1,6)-Fucosyltransferase in Cancer
Abstract
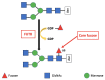
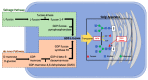
Aberrant glycosylation is a universal feature of cancer cells that can impact all steps in tumour progression from malignant transformation to metastasis and immune evasion. One key change in tumour glycosylation is altered core fucosylation. Core fucosylation is driven by fucosyltransferase 8 (FUT8), which catalyses the addition of α1,6-fucose to the innermost GlcNAc residue of N-glycans. FUT8 is frequently upregulated in cancer, and plays a critical role in immune evasion, antibody-dependent cellular cytotoxicity (ADCC), and the regulation of TGF-β, EGF, α3β1 integrin and E-Cadherin. Here, we summarise the role of FUT8 in various cancers (including lung, liver, colorectal, ovarian, prostate, breast, melanoma, thyroid, and pancreatic), discuss the potential mechanisms involved, and outline opportunities to exploit FUT8 as a critical factor in cancer therapeutics in the future.
Keywords: cancer; fucosylation; fut8; glycosylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Varki A., Lowe J.B. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2009. Biological roles of glycans. - PubMed
-
- Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., Prestegard J.H., et al. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

