The Thyroid Hormone Transporter Mct8 Restricts Cathepsin-Mediated Thyroglobulin Processing in Male Mice through Thyroid Auto-Regulatory Mechanisms That Encompass Autophagy
- PMID: 33466458
- PMCID: PMC7796480
- DOI: 10.3390/ijms22010462
The Thyroid Hormone Transporter Mct8 Restricts Cathepsin-Mediated Thyroglobulin Processing in Male Mice through Thyroid Auto-Regulatory Mechanisms That Encompass Autophagy
Abstract
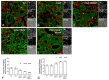
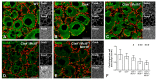
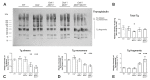
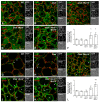
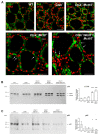
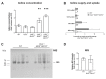
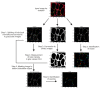
The thyroid gland is both a thyroid hormone (TH) generating as well as a TH responsive organ. It is hence crucial that cathepsin-mediated proteolytic cleavage of the precursor thyroglobulin is regulated and integrated with the subsequent export of TH into the blood circulation, which is enabled by TH transporters such as monocarboxylate transporters Mct8 and Mct10. Previously, we showed that cathepsin K-deficient mice exhibit the phenomenon of functional compensation through cathepsin L upregulation, which is independent of the canonical hypothalamus-pituitary-thyroid axis, thus, due to auto-regulation. Since these animals also feature enhanced Mct8 expression, we aimed to understand if TH transporters are part of the thyroid auto-regulatory mechanisms. Therefore, we analyzed phenotypic differences in thyroid function arising from combined cathepsin K and TH transporter deficiencies, i.e., in Ctsk-/-/Mct10-/-, Ctsk-/-/Mct8-/y, and Ctsk-/-/Mct8-/y/Mct10-/-. Despite the impaired TH export, thyroglobulin degradation was enhanced in the mice lacking Mct8, particularly in the triple-deficient genotype, due to increased cathepsin amounts and enhanced cysteine peptidase activities, leading to ongoing thyroglobulin proteolysis for TH liberation, eventually causing self-thyrotoxic thyroid states. The increased cathepsin amounts were a consequence of autophagy-mediated lysosomal biogenesis that is possibly triggered due to the stress accompanying intrathyroidal TH accumulation, in particular in the Ctsk-/-/Mct8-/y/Mct10-/- animals. Collectively, our data points to the notion that the absence of cathepsin K and Mct8 leads to excessive thyroglobulin degradation and TH liberation in a non-classical pathway of thyroid auto-regulation.
Keywords: autophagy; cathepsins; lysosomal biogenesis; monocarboxylate transporter 8; thyroid auto-regulation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






Similar articles
-
The Amino Acid Transporter Mct10/Tat1 Is Important to Maintain the TSH Receptor at Its Canonical Basolateral Localization and Assures Regular Turnover of Thyroid Follicle Cells in Male Mice.Int J Mol Sci. 2021 May 28;22(11):5776. doi: 10.3390/ijms22115776. Int J Mol Sci. 2021. PMID: 34071318 Free PMC article.
-
Interdependence of thyroglobulin processing and thyroid hormone export in the mouse thyroid gland.Eur J Cell Biol. 2017 Aug;96(5):440-456. doi: 10.1016/j.ejcb.2017.02.002. Epub 2017 Mar 6. Eur J Cell Biol. 2017. PMID: 28274595
-
Lack of L-type amino acid transporter 2 in murine thyroid tissue induces autophagy.J Mol Endocrinol. 2022 Dec 7;70(1):e220060. doi: 10.1530/JME-22-0060. Print 2023 Jan 1. J Mol Endocrinol. 2022. PMID: 36129170
-
Auto-Regulation of the Thyroid Gland Beyond Classical Pathways.Exp Clin Endocrinol Diabetes. 2020 Jun;128(6-07):437-445. doi: 10.1055/a-1080-2969. Epub 2020 Feb 19. Exp Clin Endocrinol Diabetes. 2020. PMID: 32074633 Review.
-
The pathophysiological consequences of thyroid hormone transporter deficiencies: Insights from mouse models.Biochim Biophys Acta. 2013 Jul;1830(7):3974-8. doi: 10.1016/j.bbagen.2012.04.009. Epub 2012 Apr 20. Biochim Biophys Acta. 2013. PMID: 22543196 Review.
Cited by
-
The role of transducin β-like 1 X-linked receptor 1 (TBL1XR1) in thyroid hormone metabolism and action in mice.Eur Thyroid J. 2023 Aug 14;12(5):e230077. doi: 10.1530/ETJ-23-0077. Print 2023 Oct 1. Eur Thyroid J. 2023. PMID: 37458724 Free PMC article.
-
Cryo-EM: A new dawn in thyroid biology.Mol Cell Endocrinol. 2021 Jul 1;531:111309. doi: 10.1016/j.mce.2021.111309. Epub 2021 May 5. Mol Cell Endocrinol. 2021. PMID: 33964321 Free PMC article. Review.
-
Thyroid Homeostasis: An Intricate Network of Production, Transport, Metabolism and Receptors Interaction.Int J Mol Sci. 2022 Jun 17;23(12):6751. doi: 10.3390/ijms23126751. Int J Mol Sci. 2022. PMID: 35743194 Free PMC article.
-
The Amino Acid Transporter Mct10/Tat1 Is Important to Maintain the TSH Receptor at Its Canonical Basolateral Localization and Assures Regular Turnover of Thyroid Follicle Cells in Male Mice.Int J Mol Sci. 2021 May 28;22(11):5776. doi: 10.3390/ijms22115776. Int J Mol Sci. 2021. PMID: 34071318 Free PMC article.
References
-
- Fujita H. Functional Morphology of the Thyroid. In: Jeon K.W., Friedlander M., editors. International Review of Cytology. Volume 113. Academic Press; Cambridge, MA, USA: 1988. pp. 145–185. - PubMed
-
- Brix K., Qatato M., Szumska J., Venugopalan V., Rehders M. Thyroglobulin Storage, Processing and Degradation for Thyroid Hormone Liberation. In: Luster M., Duntas L.H., Wartofsky L., editors. The Thyroid and Its Diseases. Springer; Berlin/Heidelberg, Germany: 2019. pp. 25–48. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

