BmK NSPK, a Potent Potassium Channel Inhibitor from Scorpion Buthus martensii Karsch, Promotes Neurite Outgrowth via NGF/TrkA Signaling Pathway
- PMID: 33466524
- PMCID: PMC7824859
- DOI: 10.3390/toxins13010033
BmK NSPK, a Potent Potassium Channel Inhibitor from Scorpion Buthus martensii Karsch, Promotes Neurite Outgrowth via NGF/TrkA Signaling Pathway
Abstract
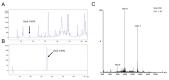
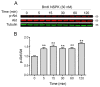
Scorpion toxins represent a variety of tools to explore molecular mechanisms and cellular signaling pathways of many biological functions. These toxins are also promising lead compounds for developing treatments for many neurological diseases. In the current study, we purified a new scorpion toxin designated as BmK NSPK (Buthus martensii Karsch neurite-stimulating peptide targeting Kv channels) from the BmK venom. The primary structure was determined using Edman degradation. BmK NSPK directly inhibited outward K+ current without affecting sodium channel activities, depolarized membrane, and increased spontaneous calcium oscillation in spinal cord neurons (SCNs) at low nanomolar concentrations. BmK NSPK produced a nonmonotonic increase on the neurite extension that peaked at ~10 nM. Mechanistic studies demonstrated that BmK NSPK increased the release of nerve growth factor (NGF). The tyrosine kinases A (TrkA) receptor inhibitor, GW 441756, eliminated the BmK NSPK-induced neurite outgrowth. BmK NSPK also increased phosphorylation levels of protein kinase B (Akt) that is the downstream regulator of TrkA receptors. These data demonstrate that BmK NSPK is a new voltage-gated potassium (Kv) channel inhibitor that augments neurite extension via NGF/TrkA signaling pathway. Kv channels may represent molecular targets to modulate SCN development and regeneration and to develop the treatments for spinal cord injury.
Keywords: nerve growth factor; neurite outgrowth; potassium channel; scorpion toxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
BmK NSP, a new sodium channel activator from Buthus martensii Karsch, promotes neurite outgrowth in primary cultured spinal cord neurons.Toxicon. 2020 Jul 30;182:13-20. doi: 10.1016/j.toxicon.2020.04.096. Epub 2020 Apr 27. Toxicon. 2020. PMID: 32353571
-
Activation of sodium channel by a novel α-scorpion toxin, BmK NT2, stimulates ERK1/2 and CERB phosphorylation through a Ca2+ dependent pathway in neocortical neurons.Int J Biol Macromol. 2017 Nov;104(Pt A):70-77. doi: 10.1016/j.ijbiomac.2017.05.163. Epub 2017 Jun 4. Int J Biol Macromol. 2017. PMID: 28591591
-
Scorpion toxins from Buthus martensii Karsch all possess a predicted alpha-tight-turn.Cell Biochem Biophys. 2003;37(3):169-86. doi: 10.1385/cbb:37:3:169. Cell Biochem Biophys. 2003. PMID: 12625626
-
An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch.Toxicon. 2002 Sep;40(9):1239-58. doi: 10.1016/s0041-0101(02)00142-3. Toxicon. 2002. PMID: 12220709 Review.
-
Anti-epileptic/pro-epileptic effects of sodium channel modulators from Buthus martensii Karsch.Sheng Li Xue Bao. 2022 Aug 25;74(4):621-632. Sheng Li Xue Bao. 2022. PMID: 35993213 Review.
Cited by
-
Activity of Potassium Channel BmK-NSPK Inhibitor Regulated by Basic Amino Acid Residues: Novel Insight into the Diverse Peptide Pharmacology.Molecules. 2025 Jan 21;30(3):450. doi: 10.3390/molecules30030450. Molecules. 2025. PMID: 39942556 Free PMC article.
-
Plantamajoside Promotes NGF/TrkA Pathway to Inhibit Neuronal Apoptosis and Improve Diabetic Peripheral Neuropathy.J Cell Mol Med. 2025 Apr;29(8):e70571. doi: 10.1111/jcmm.70571. J Cell Mol Med. 2025. PMID: 40289624 Free PMC article.
-
Animal Venoms and Their Components: Molecular Mechanisms of Action.Toxins (Basel). 2021 Jun 11;13(6):415. doi: 10.3390/toxins13060415. Toxins (Basel). 2021. PMID: 34207957 Free PMC article.
-
Bibliometric analysis of nanotechnology in spinal cord injury: current status and emerging frontiers.Front Pharmacol. 2024 Dec 11;15:1473599. doi: 10.3389/fphar.2024.1473599. eCollection 2024. Front Pharmacol. 2024. PMID: 39723251 Free PMC article.
-
Functional Characterization of a New Degradation Peptide BmTX4-P1 from Traditional Chinese Scorpion Medicinal Material.Toxins (Basel). 2023 May 15;15(5):340. doi: 10.3390/toxins15050340. Toxins (Basel). 2023. PMID: 37235373 Free PMC article.
References
-
- Zhou X. The biochemical research on scorpion venoms and their application in therapy. Progr. Biochem. Biophys. 1984;56:20–29. doi: 10.3390/toxins12050326. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- 81903826/the National Natural Science Foundation of China
- 81972960/the National Natural Science Foundation of China
- 21777192/the National Natural Science Foundation of China
- 81473539/the National Natural Science Foundation of China
- 2018ZX09101003-004-002/National Science and Technology Major Projects for Major New Drugs Innovation and Development
LinkOut - more resources
Full Text Sources
Other Literature Sources

