Botulinum Toxin: An Update on Pharmacology and Newer Products in Development
- PMID: 33466571
- PMCID: PMC7828686
- DOI: 10.3390/toxins13010058
Botulinum Toxin: An Update on Pharmacology and Newer Products in Development
Abstract
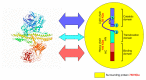
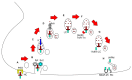
Since its introduction as a treatment for strabismus, botulinum toxin (BoNT) has had a phenomenal journey and is now recommended as first-line treatment for focal dystonia, despite short-term clinical benefits and the risks of adverse effects. To cater for the high demand across various medical specialties, at least six US Food and Drug Administration (FDA)-approved formulations of BoNT are currently available for diverse labelled indications. The toxo-pharmacological properties of these formulations are not uniform and thus should not be used interchangeably. Synthetic BoNTs and BoNTs from non-clostridial sources are not far from clinical use. Moreover, the study of mutations in naturally occurring toxins has led to modulation in the toxo-pharmacokinetic properties of BoNTs, including the duration and potency. We present an overview of the toxo-pharmacology of conventional and novel BoNT preparations, including those awaiting imminent translation from the laboratory to the clinic.
Keywords: acetylcholine; botulinum toxin; dystonia; neuromuscular blockade; recombinant botulinum toxin.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

