Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine
- PMID: 33466583
- PMCID: PMC7828743
- DOI: 10.3390/ijms22020763
Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine
Abstract
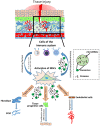
Mesenchymal stromal/stem cells (MSCs) are multipotent adult stem cells that support homeostasis during tissue regeneration. In the last decade, cell therapies based on the use of MSCs have emerged as a promising strategy in the field of regenerative medicine. Although these cells possess robust therapeutic properties that can be applied in the treatment of different diseases, variables in preclinical and clinical trials lead to inconsistent outcomes. MSC therapeutic effects result from the secretion of bioactive molecules affected by either local microenvironment or MSC culture conditions. Hence, MSC paracrine action is currently being explored in several clinical settings either using a conditioned medium (CM) or MSC-derived exosomes (EXOs), where these products modulate tissue responses in different types of injuries. In this scenario, MSC paracrine mechanisms provide a promising framework for enhancing MSC therapeutic benefits, where the composition of secretome can be modulated by priming of the MSCs. In this review, we examine the literature on the priming of MSCs as a tool to enhance their therapeutic properties applicable to the main processes involved in tissue regeneration, including the reduction of fibrosis, the immunomodulation, the stimulation of angiogenesis, and the stimulation of resident progenitor cells, thereby providing new insights for the therapeutic use of MSCs-derived products.
Keywords: Mesenchymal stromal/stem cells; cell-free therapies; paracrine mechanism; priming; regenerative medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Itkin T., Ludin A., Gradus B., Gur-Cohen S., Kalinkovich A., Schajnovitz A., Ovadya Y., Kollet O., Canaani J., Shezen E., et al. FGF-2 expands murine hematopoietic stem and progenitor cells via proliferation of stromal cells, c-Kit activation, and CXCL12 down-regulation. Blood. 2012;120:1843–1855. doi: 10.1182/blood-2011-11-394692. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

