Effects of 6,8-Diprenylgenistein on VEGF-A-Induced Lymphangiogenesis and Lymph Node Metastasis in an Oral Cancer Sentinel Lymph Node Animal Model
- PMID: 33466636
- PMCID: PMC7828717
- DOI: 10.3390/ijms22020770
Effects of 6,8-Diprenylgenistein on VEGF-A-Induced Lymphangiogenesis and Lymph Node Metastasis in an Oral Cancer Sentinel Lymph Node Animal Model
Abstract
Background: The major determining factor of prognosis of oral squamous cell carcinoma is cervical lymph node metastasis. 6,8-Diprenylgenistein (6,8-DG), an isoflavonoid isolated from Cudrania tricuspidata has been reported to have anti-microbial and anti-obesity activities. However, its effects on lymphangiogenesis and lymph node metastasis in oral cancer have not yet been reported.
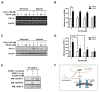
Methods: To investigate the in vitro inhibitory effects of 6,8-DG on VEGF-A-induced lymphangiogenesis, we performed the proliferation, tube formation, and migration assay using human lymphatic microvascular endothelial cells (HLMECs). RT-PCR, Western blot, immunoprecipitation, ELISA and co-immunoprecipitation assays were used to investigate the expression levels of proteins, and mechanism of 6,8-DG. The in vivo inhibitory effects of 6,8-DG were investigated using an oral cancer sentinel lymph node (OCSLN) animal model.
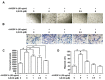
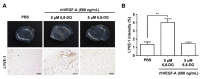
Results: 6,8-DG inhibited the proliferation, migration and tube formation of rhVEGF-A treated HLMECs. In addition, the in vivo lymphatic vessel formation stimulated by rhVEGF-A was significantly reduced by 6,8-DG. 6,8-DG inhibited the expression of VEGF-A rather than other lymphangiogenic factors in CoCl2-treated SCCVII cells. 6,8-DG inhibited the expression and activation of VEGFR-2 stimulated by rhVEGF-A in HLMECs. Also, 6,8-DG inhibited the activation of the lymphangiogenesis-related downstream signaling factors such as FAK, PI3K, AKT, p38, and ERK in rhVEGF-A-treated HLMECs. Additionally, 6,8-DG inhibited the expression of the hypoxia-inducible factor (HIF-1α), which is involved in the expression of VEGF-A in CoCl2-treated SCCVII cells, and 6,8-DG inhibited VEGF-A signaling via interruption of the binding of VEGF-A and VEGFR-2 in HLMECs. In the VEGF-A-induced OCSLN animal model, we confirmed that 6,8-DG suppressed tumor-induced lymphangiogenesis and SLN metastasis.
Conclusion: These data suggest that 6,8-DG inhibits VEGF-A-induced lymphangiogenesis and lymph node metastasis in vitro and in vivo. Furthermore, the inhibitory effects of 6,8-DG are probably mediated by inhibition of VEGF-A expression in cancer cells and suppression of the VEGF-A/VEGFR-2 signaling pathway in HLMEC. Thus, 6,8-DG could be novel and valuable therapeutic agents for metastasis prevention and treatment of oral cancer.
Keywords: 6,8-diprenylgenistein; VEGF-A-induced lymphangiogenesis; oral cancer; sentinel lymph node metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
3-O-Acetyloleanolic acid inhibits VEGF-A-induced lymphangiogenesis and lymph node metastasis in an oral cancer sentinel lymph node animal model.BMC Cancer. 2018 Jul 5;18(1):714. doi: 10.1186/s12885-018-4630-0. BMC Cancer. 2018. PMID: 29976150 Free PMC article.
-
Recombinant canstatin inhibits VEGF-A-induced lymphangiogenesis and metastasis in an oral squamous cell carcinoma SCC-VII animal model.Cancer Med. 2016 Oct;5(10):2977-2988. doi: 10.1002/cam4.866. Epub 2016 Sep 20. Cancer Med. 2016. PMID: 27650585 Free PMC article.
-
Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3.Breast Cancer Res. 2011 Jun 21;13(3):R66. doi: 10.1186/bcr2903. Breast Cancer Res. 2011. PMID: 21693010 Free PMC article.
-
Pathways targeting tumor lymphangiogenesis.Clin Cancer Res. 2006 Dec 1;12(23):6865-8. doi: 10.1158/1078-0432.CCR-06-1800. Clin Cancer Res. 2006. PMID: 17145802 Review.
-
Molecular control of lymphatic metastasis.Ann N Y Acad Sci. 2008;1131:225-34. doi: 10.1196/annals.1413.020. Ann N Y Acad Sci. 2008. PMID: 18519975 Review.
Cited by
-
Effects of Allium sativum Stem Extract on Growth and Migration in Melanoma Cells through Inhibition of VEGF, MMP-2, and MMP-9 Genes Expression.Molecules. 2021 Dec 21;27(1):21. doi: 10.3390/molecules27010021. Molecules. 2021. PMID: 35011253 Free PMC article.
-
Epithelial-to-mesenchymal transition (EMT) and cancer metastasis: the status quo of methods and experimental models 2025.Mol Cancer. 2025 Jun 7;24(1):167. doi: 10.1186/s12943-025-02338-2. Mol Cancer. 2025. PMID: 40483504 Free PMC article. Review.
-
EGR1 Enhances Lymphangiogenesis via SOX18-Mediated Activation of JAK2/STAT3 Pathway.Comput Math Methods Med. 2022 Feb 12;2022:6448724. doi: 10.1155/2022/6448724. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Jun 28;2023:9796376. doi: 10.1155/2023/9796376. PMID: 35190753 Free PMC article. Retracted.
-
The exploration of new biomarkers for oral cancer through the ceRNA network and immune microenvironment analysis.Medicine (Baltimore). 2022 Dec 9;101(49):e32249. doi: 10.1097/MD.0000000000032249. Medicine (Baltimore). 2022. PMID: 36626444 Free PMC article.
-
Pharmacological Approaches to Attenuate Inflammation and Obesity with Natural Products Formulations by Regulating the Associated Promoting Molecular Signaling Pathways.Biomed Res Int. 2021 Nov 12;2021:2521273. doi: 10.1155/2021/2521273. eCollection 2021. Biomed Res Int. 2021. PMID: 34812408 Free PMC article.
References
-
- Wang W.M., Zhao Z.L., Ma S.R., Yu G.T., Liu B., Zhang L., Zhang W.F., Kulkarni A.B., Sun Z.J., Zhao Y.F. Epidermal growth factor receptor inhibition reduces angiogenesis via hypoxia-inducible factor-1α and notch1 in head neck squamous cell carcinoma. PLoS ONE. 2015;10:e0119723. doi: 10.1371/journal.pone.0119723. - DOI - PMC - PubMed
-
- Wakisaka N., Hasegawa Y., Yoshimoto S., Miura K., Shiotani A., Yokoyama J., Sugasawa M., Moriyama-Kita M., Endo K., Yoshizaki T. Primary tumor-secreted lymphangiogenic factors induce pre-metastatic lymphvascular niche formation at sentinel lymph nodes in oral squamous cell carcinoma. PLoS ONE. 2015;10:e0144056. doi: 10.1371/journal.pone.0144056. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

