A Neurotoxic Snake Venom without Phospholipase A2: Proteomics and Cross-Neutralization of the Venom from Senegalese Cobra, Naja senegalensis (Subgenus: Uraeus)
- PMID: 33466660
- PMCID: PMC7828783
- DOI: 10.3390/toxins13010060
A Neurotoxic Snake Venom without Phospholipase A2: Proteomics and Cross-Neutralization of the Venom from Senegalese Cobra, Naja senegalensis (Subgenus: Uraeus)
Abstract
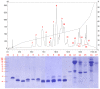
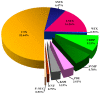
The Senegalese cobra, Naja senegalensis, is a non-spitting cobra species newly erected from the Naja haje complex. Naja senegalensis causes neurotoxic envenomation in Western Africa but its venom properties remain underexplored. Applying a protein decomplexation proteomic approach, this study unveiled the unique complexity of the venom composition. Three-finger toxins constituted the major component, accounting for 75.91% of total venom proteins. Of these, cardiotoxin/cytotoxin (~53%) and alpha-neurotoxins (~23%) predominated in the venom proteome. Phospholipase A2, however, was not present in the venom, suggesting a unique snake venom phenotype found in this species. The venom, despite the absence of PLA2, is highly lethal with an intravenous LD50 of 0.39 µg/g in mice, consistent with the high abundance of alpha-neurotoxins (predominating long neurotoxins) in the venom. The hetero-specific VINS African Polyvalent Antivenom (VAPAV) was immunoreactive to the venom, implying conserved protein antigenicity in the venoms of N. senegalensis and N. haje. Furthermore, VAPAV was able to cross-neutralize the lethal effect of N. senegalensis venom but the potency was limited (0.59 mg venom completely neutralized per mL antivenom, or ~82 LD50 per ml of antivenom). The efficacy of antivenom should be further improved to optimize the treatment of cobra bite envenomation in Africa.
Keywords: Naja (Uraeus) senegalensis; Naja haje complex; antivenom neutralization; immunoreactivity; snakebite envenomation; venomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Snake Venomics and Antivenomics of Cape Cobra (Naja nivea) from South Africa: Insights into Venom Toxicity and Cross-Neutralization Activity.Toxins (Basel). 2022 Dec 7;14(12):860. doi: 10.3390/toxins14120860. Toxins (Basel). 2022. PMID: 36548757 Free PMC article.
-
Elucidating the biogeographical variation of the venom of Naja naja (spectacled cobra) from Pakistan through a venom-decomplexing proteomic study.J Proteomics. 2018 Mar 20;175:156-173. doi: 10.1016/j.jprot.2017.12.012. Epub 2017 Dec 24. J Proteomics. 2018. PMID: 29278784
-
Venomics of Naja sputatrix, the Javan spitting cobra: A short neurotoxin-driven venom needing improved antivenom neutralization.J Proteomics. 2017 Mar 22;157:18-32. doi: 10.1016/j.jprot.2017.01.018. Epub 2017 Jan 31. J Proteomics. 2017. PMID: 28159706
-
A comparison of the venom proteomes and potential therapeutics of 3 African naja subgenera.Toxicon. 2024 Jul;245:107792. doi: 10.1016/j.toxicon.2024.107792. Epub 2024 Jun 3. Toxicon. 2024. PMID: 38838860 Review.
-
A Review of the Proteomic Profiling of African Viperidae and Elapidae Snake Venoms and Their Antivenom Neutralisation.Toxins (Basel). 2022 Oct 22;14(11):723. doi: 10.3390/toxins14110723. Toxins (Basel). 2022. PMID: 36355973 Free PMC article. Review.
Cited by
-
Egyptian cobra (Naja haje haje) venom phospholipase A2: a promising antiviral agent with potent virucidal activity against simian rotavirus and bovine coronavirus.Arch Microbiol. 2022 Jul 27;204(8):526. doi: 10.1007/s00203-022-03139-7. Arch Microbiol. 2022. PMID: 35895237 Free PMC article.
-
Equatorial Spitting Cobra (Naja sumatrana) from Malaysia (Negeri Sembilan and Penang), Southern Thailand, and Sumatra: Comparative Venom Proteomics, Immunoreactivity and Cross-Neutralization by Antivenom.Toxins (Basel). 2022 Jul 29;14(8):522. doi: 10.3390/toxins14080522. Toxins (Basel). 2022. PMID: 36006183 Free PMC article.
-
Proteomic Investigation of Cape Cobra (Naja nivea) Venom Reveals First Evidence of Quaternary Protein Structures.Toxins (Basel). 2024 Jan 23;16(2):63. doi: 10.3390/toxins16020063. Toxins (Basel). 2024. PMID: 38393141 Free PMC article.
-
Intrageneric cross-reactivity of monospecific rabbit antisera against venoms of the medically most important Naja spp. African snakes.PLoS Negl Trop Dis. 2023 Aug 15;17(8):e0011545. doi: 10.1371/journal.pntd.0011545. eCollection 2023 Aug. PLoS Negl Trop Dis. 2023. PMID: 37582064 Free PMC article.
-
Three finger toxins of elapids: structure, function, clinical applications and its inhibitors.Mol Divers. 2024 Oct;28(5):3409-3426. doi: 10.1007/s11030-023-10734-3. Epub 2023 Sep 25. Mol Divers. 2024. PMID: 37749455 Review.
References
-
- Williams D.J., Faiz M.A., Abela-Ridder B., Ainsworth S., Bulfone T.C., Nickerson A.D., Habib A.G., Junghanss T., Fan H.W., Turner M., et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl. Trop. Dis. 2019;13:e0007059. doi: 10.1371/journal.pntd.0007059. - DOI - PMC - PubMed
-
- WHO Venomous Snakes and Antivenoms Search Interface. [(accessed on 2 April 2020)]; Available online: http://apps.who.int/bloodproducts/snakeantivenoms/database/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

