Mechanisms of the Morphological Plasticity Induced by Phytohormones and the Environment in Plants
- PMID: 33466729
- PMCID: PMC7828791
- DOI: 10.3390/ijms22020765
Mechanisms of the Morphological Plasticity Induced by Phytohormones and the Environment in Plants
Abstract
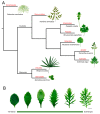
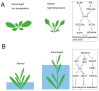
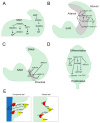
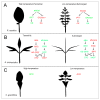
Plants adapt to environmental changes by regulating their development and growth. As an important interface between plants and their environment, leaf morphogenesis varies between species, populations, or even shows plasticity within individuals. Leaf growth is dependent on many environmental factors, such as light, temperature, and submergence. Phytohormones play key functions in leaf development and can act as molecular regulatory elements in response to environmental signals. In this review, we discuss the current knowledge on the effects of different environmental factors and phytohormone pathways on morphological plasticity and intend to summarize the advances in leaf development. In addition, we detail the molecular mechanisms of heterophylly, the representative of leaf plasticity, providing novel insights into phytohormones and the environmental adaptation in plants.
Keywords: environment; leaf; molecular mechanism; morphological plasticity; phytohormones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Synergistic Interaction of Phytohormones in Determining Leaf Angle in Crops.Int J Mol Sci. 2020 Jul 17;21(14):5052. doi: 10.3390/ijms21145052. Int J Mol Sci. 2020. PMID: 32709150 Free PMC article. Review.
-
Molecular Mechanisms of Leaf Morphogenesis.Mol Plant. 2018 Sep 10;11(9):1117-1134. doi: 10.1016/j.molp.2018.06.006. Epub 2018 Jun 28. Mol Plant. 2018. PMID: 29960106 Review.
-
Water-Wisteria as an ideal plant to study heterophylly in higher aquatic plants.Plant Cell Rep. 2017 Aug;36(8):1225-1236. doi: 10.1007/s00299-017-2148-6. Epub 2017 May 2. Plant Cell Rep. 2017. PMID: 28466187
-
Developmental transitions: integrating environmental cues with hormonal signaling in the chromatin landscape in plants.Genome Biol. 2017 May 10;18(1):88. doi: 10.1186/s13059-017-1228-9. Genome Biol. 2017. PMID: 28490341 Free PMC article. Review.
-
The phytohormone signal network regulating elongation growth during shade avoidance.J Exp Bot. 2010 Jun;61(11):2889-903. doi: 10.1093/jxb/erq147. Epub 2010 May 25. J Exp Bot. 2010. PMID: 20501746 Review.
Cited by
-
Adaptation to Climate Change in Viticulture: The Role of Varietal Selection-A Review.Plants (Basel). 2025 Jan 2;14(1):104. doi: 10.3390/plants14010104. Plants (Basel). 2025. PMID: 39795365 Free PMC article. Review.
-
Comparative Transcriptome Analysis of Gene Expression Between Female and Monoecious Spinacia oleracea L.Genes (Basel). 2024 Dec 27;16(1):24. doi: 10.3390/genes16010024. Genes (Basel). 2024. PMID: 39858571 Free PMC article.
-
Auxin and Cytokinin Interplay during Leaf Morphogenesis and Phyllotaxy.Plants (Basel). 2021 Aug 21;10(8):1732. doi: 10.3390/plants10081732. Plants (Basel). 2021. PMID: 34451776 Free PMC article. Review.
-
Arsenic Toxicity-Induced Physiological and Metabolic Changes in the Shoots of Pteris cretica and Spinacia oleracea.Plants (Basel). 2021 Sep 25;10(10):2009. doi: 10.3390/plants10102009. Plants (Basel). 2021. PMID: 34685818 Free PMC article.
-
Cooperation of an external carbonic anhydrase and HCO3- transporter supports underwater photosynthesis in submerged leaves of the amphibious plant Hygrophila difformis.Ann Bot. 2024 Apr 10;133(2):287-304. doi: 10.1093/aob/mcad161. Ann Bot. 2024. PMID: 37832038 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

