Cannabis sativa L. as a Natural Drug Meeting the Criteria of a Multitarget Approach to Treatment
- PMID: 33466734
- PMCID: PMC7830475
- DOI: 10.3390/ijms22020778
Cannabis sativa L. as a Natural Drug Meeting the Criteria of a Multitarget Approach to Treatment
Abstract
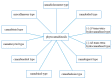
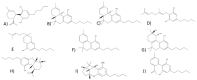
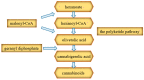
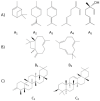
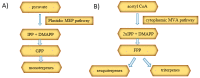
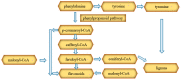
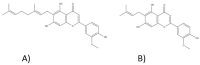
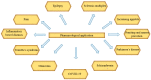
Cannabis sativa L. turned out to be a valuable source of chemical compounds of various structures, showing pharmacological activity. The most important groups of compounds include phytocannabinoids and terpenes. The pharmacological activity of Cannabis (in epilepsy, sclerosis multiplex (SM), vomiting and nausea, pain, appetite loss, inflammatory bowel diseases (IBDs), Parkinson's disease, Tourette's syndrome, schizophrenia, glaucoma, and coronavirus disease 2019 (COVID-19)), which has been proven so far, results from the affinity of these compounds predominantly for the receptors of the endocannabinoid system (the cannabinoid receptor type 1 (CB1), type two (CB2), and the G protein-coupled receptor 55 (GPR55)) but, also, for peroxisome proliferator-activated receptor (PPAR), glycine receptors, serotonin receptors (5-HT), transient receptor potential channels (TRP), and GPR, opioid receptors. The synergism of action of phytochemicals present in Cannabis sp. raw material is also expressed in their increased bioavailability and penetration through the blood-brain barrier. This review provides an overview of phytochemistry and pharmacology of compounds present in Cannabis extracts in the context of the current knowledge about their synergistic actions and the implications of clinical use in the treatment of selected diseases.
Keywords: Cannabis; multitarget; phytocannabinoids (THC and CBD); receptors; terpenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Thatoi H., Patra J.K. Biotechnology and pharmacological evaluation of medicinal plants: An overview. J. Herbs. Spices Med. Plants. 2011;17:214–248. doi: 10.1080/10496475.2011.602471. - DOI
-
- Chida N. Chemistry of Opioids. Springer; Berlin/Heidelberg, Germany: 2010. Recent advances in the synthesis of morphine and related alkaloids; pp. 1–28. - PubMed
-
- Gulland J.M.R., Mem R. Constitution of codeine and thebaine. Proc. Manch. Lit. Phil. Soc. 1925;69:79–86.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

