Amikacin or Vancomycin Exposure Alters the Postnatal Serum Creatinine Dynamics in Extreme Low Birth Weight Neonates
- PMID: 33466764
- PMCID: PMC7830583
- DOI: 10.3390/ijerph18020662
Amikacin or Vancomycin Exposure Alters the Postnatal Serum Creatinine Dynamics in Extreme Low Birth Weight Neonates
Abstract
Background: Disentangling renal adverse drug reactions from confounders remains a major challenge to assess causality and severity in neonates, with additional limitations related to the available tools (modified Kidney Disease Improving Global Outcome, or Division of Microbiology and Infectious Diseases pediatric toxicity table). Vancomycin and amikacin are nephrotoxic while still often prescribed in neonates. We selected these compounds to assess their impact on creatinine dynamics as a sensitive tool to detect a renal impairment signal.
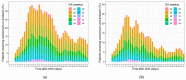
Methods: A recently developed dynamical model that characterized serum creatinine concentrations of 217 extremely low birth weight (<1000 g, ELBW) neonates (4036 observations) was enhanced with data on vancomycin and/or amikacin exposure to identify a potential effect of antibiotic exposure by nonlinear mixed-effects modelling.
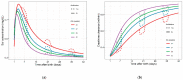
Results: Seventy-seven percent of ELBW patients were exposed to either vancomycin or amikacin. Antibiotic exposure resulted in a modest increase in serum creatinine and a transient decrease in creatinine clearance. The serum creatinine increase was dependent on gestational age, illustrated by a decrease with 56% in difference in serum creatinine between a 24 or 32-week old neonate, when exposed in the 3rd week after birth.
Conclusions: A previously described model was used to explore and quantify the impact of amikacin or vancomycin exposure on creatinine dynamics. Such tools serve to explore minor changes, or compare minor differences between treatment modalities.
Keywords: acute kidney injury; adverse drug reaction; amikacin; creatinine; renal impairment; vancomycin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Pharmacovigilance of nephrotoxic drugs in neonates: the Pottel method for acute kidney injury detection in ELBW neonates.Pediatr Nephrol. 2024 Aug;39(8):2525-2532. doi: 10.1007/s00467-024-06335-3. Epub 2024 Mar 25. Pediatr Nephrol. 2024. PMID: 38526762 Free PMC article.
-
Creatinine Trends to Detect Ibuprofen-Related Maturational Adverse Drug Events in Neonatal Life: A Simulation Study for the ELBW Newborn.Front Pharmacol. 2021 Jan 25;11:610294. doi: 10.3389/fphar.2020.610294. eCollection 2020. Front Pharmacol. 2021. PMID: 33569003 Free PMC article.
-
Renal Precision Medicine in Neonates and Acute Kidney Injury: How to Convert a Cloud of Creatinine Observations to Support Clinical Decisions.Front Pediatr. 2020 Jul 28;8:366. doi: 10.3389/fped.2020.00366. eCollection 2020. Front Pediatr. 2020. PMID: 32850523 Free PMC article. Review.
-
Renal drug clearance in preterm neonates: relation to prenatal growth.Ther Drug Monit. 2007 Jun;29(3):284-91. doi: 10.1097/FTD.0b013e31806db3f5. Ther Drug Monit. 2007. PMID: 17529884
-
Vancomycin does not enhance amikacin-induced tubular nephrotoxicity in children.Pediatr Infect Dis J. 1989 May;8(5):278-82. Pediatr Infect Dis J. 1989. PMID: 2657616 Review.
Cited by
-
Plasma concentration and eGFR in preterm and term neonates receiving gentamicin or successive amikacin therapy.BMC Pediatr. 2023 Jan 16;23(1):24. doi: 10.1186/s12887-023-03834-4. BMC Pediatr. 2023. PMID: 36647065 Free PMC article.
-
Pharmacovigilance of nephrotoxic drugs in neonates: the Pottel method for acute kidney injury detection in ELBW neonates.Pediatr Nephrol. 2024 Aug;39(8):2525-2532. doi: 10.1007/s00467-024-06335-3. Epub 2024 Mar 25. Pediatr Nephrol. 2024. PMID: 38526762 Free PMC article.
References
-
- Van den Anker J.N., McCune S., Annaert P., Baer G.R., Mulugeta Y., Abdelrahman R., Wu K., Krudys K.M., Fisher J., Slikker W., et al. Approaches to Dose Finding in Neonates, Illustrating the Variability between Neonatal Drug Development Programs. Pharmaceutics. 2020;12:685. doi: 10.3390/pharmaceutics12070685. - DOI - PMC - PubMed
-
- Salaets T., Turner M.A., Short M., Ward R.M., Hokuto I., Ariagno R.L., Klein A., Beauman S., Wade K., Thomson M., et al. Development of a neonatal adverse event severity scale through a Delphi consensus approach. Arch. Dis. Child. 2019;104:1167–1173. doi: 10.1136/archdischild-2019-317399. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

