Molecular and Clinical Features of EGFR-TKI-Associated Lung Injury
- PMID: 33466795
- PMCID: PMC7829873
- DOI: 10.3390/ijms22020792
Molecular and Clinical Features of EGFR-TKI-Associated Lung Injury
Abstract
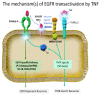
The tyrosine kinase activity of epidermal growth factor receptors (EGFRs) plays critical roles in cell proliferation, regeneration, tumorigenesis, and anticancer resistance. Non-small-cell lung cancer patients who responded to EGFR-tyrosine kinase inhibitors (EGFR-TKIs) and obtained survival benefits had somatic EGFR mutations. EGFR-TKI-related adverse events (AEs) are usually tolerable and manageable, although serious AEs, including lung injury (specifically, interstitial lung disease (ILD), causing 58% of EGFR-TKI treatment-related deaths), occur infrequently. The etiopathogenesis of EGFR-TKI-induced ILD remains unknown. Risk factors, such as tobacco exposure, pre-existing lung fibrosis, chronic obstructive pulmonary disease, and poor performance status, indicate that lung inflammatory circumstances may worsen with EGFR-TKI treatment because of impaired epithelial healing of lung injuries. There is limited evidence from preclinical and clinical studies of the mechanisms underlying EGFR-TKI-induced ILD in the available literature. Herein, we evaluated the relationship between EGFR-TKIs and AEs, especially ILD. Recent reports on mechanisms inducing lung injury or resistance in cytokine-rich circumstances were reviewed. We discussed the relevance of cytotoxic agents or immunotherapeutic agents in combination with EGFR-TKIs as a potential mechanism of EGFR-TKI-related lung injury and reviewed recent developments in diagnostics and therapeutics that facilitate recovery from lung injury or overcoming resistance to anti-EGFR treatment.
Keywords: EGFR-TKIs; TNF; inflammation; lung injury.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the choice of research project; design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Proto C., Ferrara R., Signorelli D., Lo Russo G., Galli G., Imbimbo M., Prelaj A., Zilembo N., Ganzinelli M., Pallavicini L.M., et al. Choosing wisely first line immunotherapy in non-small cell lung cancer (NSCLC): What to add and what to leave out. Cancer Treat. Rev. 2019;75:39–51. doi: 10.1016/j.ctrv.2019.03.004. - DOI - PubMed
-
- Yatabe Y., Kerr K.M., Utomo A., Rajadurai P., Tran V.K., Du X., Chou T.Y., Enriquez M.L., Lee G.K., Iqbal J., et al. EGFR mutation testing practices within the Asia Pacific region: Results of a multicenter diagnostic survey. J. Thorac. Oncol. 2015;10:438–445. doi: 10.1097/JTO.0000000000000422. - DOI - PMC - PubMed
-
- Zhou C., Wu Y.L., Chen G., Feng J., Liu X.Q., Wang C., Zhang S., Wang J., Zhou S., Ren S., et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous