Radiochemistry, Production Processes, Labeling Methods, and ImmunoPET Imaging Pharmaceuticals of Iodine-124
- PMID: 33466827
- PMCID: PMC7830191
- DOI: 10.3390/molecules26020414
Radiochemistry, Production Processes, Labeling Methods, and ImmunoPET Imaging Pharmaceuticals of Iodine-124
Abstract
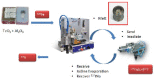
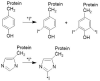
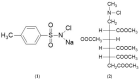
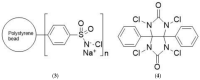
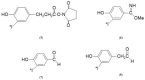
Target-specific biomolecules, monoclonal antibodies (mAb), proteins, and protein fragments are known to have high specificity and affinity for receptors associated with tumors and other pathological conditions. However, the large biomolecules have relatively intermediate to long circulation half-lives (>day) and tumor localization times. Combining superior target specificity of mAbs and high sensitivity and resolution of the PET (Positron Emission Tomography) imaging technique has created a paradigm-shifting imaging modality, ImmunoPET. In addition to metallic PET radionuclides, 124I is an attractive radionuclide for radiolabeling of mAbs as potential immunoPET imaging pharmaceuticals due to its physical properties (decay characteristics and half-life), easy and routine production by cyclotrons, and well-established methodologies for radioiodination. The objective of this report is to provide a comprehensive review of the physical properties of iodine and iodine radionuclides, production processes of 124I, various 124I-labeling methodologies for large biomolecules, mAbs, and the development of 124I-labeled immunoPET imaging pharmaceuticals for various cancer targets in preclinical and clinical environments. A summary of several production processes, including 123Te(d,n)124I, 124Te(d,2n)124I, 121Sb(α,n)124I, 123Sb(α,3n)124I, 123Sb(3He,2n)124I, natSb(α, xn)124I, natSb(3He,n)124I reactions, a detailed overview of the 124Te(p,n)124I reaction (including target selection, preparation, processing, and recovery of 124I), and a fully automated process that can be scaled up for GMP (Good Manufacturing Practices) production of large quantities of 124I is provided. Direct, using inorganic and organic oxidizing agents and enzyme catalysis, and indirect, using prosthetic groups, 124I-labeling techniques have been discussed. Significant research has been conducted, in more than the last two decades, in the development of 124I-labeled immunoPET imaging pharmaceuticals for target-specific cancer detection. Details of preclinical and clinical evaluations of the potential 124I-labeled immunoPET imaging pharmaceuticals are described here.
Keywords: 124I-labeled monoclonal antibodies; PET; cancer; immunoPET imaging pharmaceuticals; positron emission tomography; production processes; radiolabeling; radiotracers; target-specific biomolecules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

