Inhibition of Orexin/Hypocretin Neurons Ameliorates Elevated Physical Activity and Energy Expenditure in the A53T Mouse Model of Parkinson's Disease
- PMID: 33466831
- PMCID: PMC7830608
- DOI: 10.3390/ijms22020795
Inhibition of Orexin/Hypocretin Neurons Ameliorates Elevated Physical Activity and Energy Expenditure in the A53T Mouse Model of Parkinson's Disease
Abstract
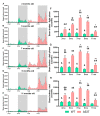
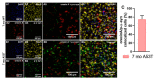
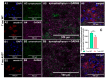
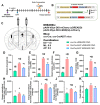
Aside from the classical motor symptoms, Parkinson's disease also has various non-classical symptoms. Interestingly, orexin neurons, involved in the regulation of exploratory locomotion, spontaneous physical activity, and energy expenditure, are affected in Parkinson's. In this study, we hypothesized that Parkinson's-disease-associated pathology affects orexin neurons and therefore impairs functions they regulate. To test this, we used a transgenic animal model of Parkinson's, the A53T mouse. We measured body composition, exploratory locomotion, spontaneous physical activity, and energy expenditure. Further, we assessed alpha-synuclein accumulation, inflammation, and astrogliosis. Finally, we hypothesized that chemogenetic inhibition of orexin neurons would ameliorate observed impairments in the A53T mice. We showed that aging in A53T mice was accompanied by reductions in fat mass and increases in exploratory locomotion, spontaneous physical activity, and energy expenditure. We detected the presence of alpha-synuclein accumulations in orexin neurons, increased astrogliosis, and microglial activation. Moreover, loss of inhibitory pre-synaptic terminals and a reduced number of orexin cells were observed in A53T mice. As hypothesized, this chemogenetic intervention mitigated the behavioral disturbances induced by Parkinson's disease pathology. This study implicates the involvement of orexin in early Parkinson's-disease-associated impairment of hypothalamic-regulated physiological functions and highlights the importance of orexin neurons in Parkinson's disease symptomology.
Keywords: Parkinson’s disease; neuromodulation; orexin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Early Sociability and Social Memory Impairment in the A53T Mouse Model of Parkinson's Disease Are Ameliorated by Chemogenetic Modulation of Orexin Neuron Activity.Mol Neurobiol. 2019 Dec;56(12):8435-8450. doi: 10.1007/s12035-019-01682-x. Epub 2019 Jun 27. Mol Neurobiol. 2019. PMID: 31250383 Free PMC article.
-
Pharmacological and chemogenetic orexin/hypocretin intervention ameliorates Hipp-dependent memory impairment in the A53T mice model of Parkinson's disease.Mol Brain. 2019 Oct 30;12(1):87. doi: 10.1186/s13041-019-0514-8. Mol Brain. 2019. PMID: 31666100 Free PMC article.
-
Chemogenetic Modulation of Orexin Neurons Reverses Changes in Anxiety and Locomotor Activity in the A53T Mouse Model of Parkinson's Disease.Front Neurosci. 2019 Jul 30;13:702. doi: 10.3389/fnins.2019.00702. eCollection 2019. Front Neurosci. 2019. PMID: 31417337 Free PMC article.
-
Orexin pathway in Parkinson's disease: a review.Mol Biol Rep. 2023 Jul;50(7):6107-6120. doi: 10.1007/s11033-023-08459-5. Epub 2023 May 8. Mol Biol Rep. 2023. PMID: 37155018 Review.
-
Transgenic mice with human mutant genes causing Parkinson's disease and amyotrophic lateral sclerosis provide common insight into mechanisms of motor neuron selective vulnerability to degeneration.Rev Neurosci. 2007;18(2):115-36. doi: 10.1515/revneuro.2007.18.2.115. Rev Neurosci. 2007. PMID: 17593875 Review.
Cited by
-
Peiminine Reduces ARTS-Mediated Degradation of XIAP by Modulating the PINK1/Parkin Pathway to Ameliorate 6-Hydroxydopamine Toxicity and α-Synuclein Accumulation in Parkinson's Disease Models In Vivo and In Vitro.Int J Mol Sci. 2021 Sep 23;22(19):10240. doi: 10.3390/ijms221910240. Int J Mol Sci. 2021. PMID: 34638579 Free PMC article.
-
Orexin, serotonin, and energy balance.WIREs Mech Dis. 2022 Jan;14(1):e1536. doi: 10.1002/wsbm.1536. Epub 2021 Sep 15. WIREs Mech Dis. 2022. PMID: 35023323 Free PMC article. Review.
-
Interactions between Lateral Hypothalamic Orexin and Dorsal Raphe Circuitry in Energy Balance.Brain Sci. 2024 May 7;14(5):464. doi: 10.3390/brainsci14050464. Brain Sci. 2024. PMID: 38790443 Free PMC article.
-
Physiological Role of Orexinergic System for Health.Int J Environ Res Public Health. 2022 Jul 8;19(14):8353. doi: 10.3390/ijerph19148353. Int J Environ Res Public Health. 2022. PMID: 35886210 Free PMC article. Review.
-
Potential Crosstalk Between Parkinson's Disease and Energy Metabolism.Aging Dis. 2021 Dec 1;12(8):2003-2015. doi: 10.14336/AD.2021.0422. eCollection 2021 Dec. Aging Dis. 2021. PMID: 34881082 Free PMC article. Review.
References
-
- Moisan F., Kab S., Mohamed F., Canonico M., Guern M.L., Quintin C., Carcaillon L., Nicolau J., Duport N., Singh-Manoux A., et al. Parkinson Disease Male-to-Female Ratios Increase with Age: French Nationwide Study and Meta-Analysis. J. Neurol. Neurosurg. Psychiatry. 2016;87:952–957. doi: 10.1136/jnnp-2015-312283. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

