Four Novel Phenanthrene Derivatives with α-Glucosidase Inhibitory Activity from Gastrochilus bellinus
- PMID: 33466863
- PMCID: PMC7830893
- DOI: 10.3390/molecules26020418
Four Novel Phenanthrene Derivatives with α-Glucosidase Inhibitory Activity from Gastrochilus bellinus
Abstract
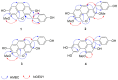
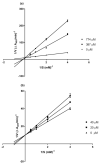
Four new phenanthrene derivatives, gastrobellinols A-D (1-4), were isolated from the methanolic extract of Gastrochilus bellinus (Rchb.f.) Kuntze, along with eleven known phenolic compounds including agrostophyllin (5), agrostophyllidin (6), coniferyl aldehyde (7), 4-hydroxybenzaldehyde (8), agrostophyllone (9), gigantol (10), 4-(methoxylmethyl)phenol (11), syringaldehyde (12), 1-(4'-hydroxybenzyl)-imbricartin (13), 6-methoxycoelonin (14), and imbricatin (15). Their structures were determined by spectroscopic methods. Each isolate was evaluated for α-glucosidase inhibitory activity. Compounds 1, 2, 3, 7, 9, 13, and 15 showed higher activity than the drug acarbose. Gastrobellinol C (3) exhibited the strongest α-glucosidase inhibition with an IC50 value of 45.92 μM. A kinetic study of 3 showed competitive inhibition on the α-glucosidase enzyme. This is the first report on the phytochemical constituents and α-glucosidase inhibitory activity of G. bellinus.
Keywords: Gastrochilus bellinus; Orchidaceae; gastrobellinol; phenanthrene derivatives; α-glucosidase inhibition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- San H.T., Boonsnongcheep P., Putalun W., Mekboonsonglarp W., Sritularak B., Likhitwitayawuid K. α-Glucosidase inhibitory and glucose uptake stimulatory effects of phenolic compounds from Dendrobium christyanum. Nat. Prod. Commun. 2020;15:1–8. doi: 10.1177/1934578X20913453. - DOI
-
- Inthongkaew P., Chatsumpun N., Supasuteekul C., Kitisripanya T., Putalun W., Likhitwitayawuid K., Sritularak B. α-Glucosidase and pancreatic lipase inhibitory activities and glucose uptake stimulatory effect of phenolic compounds from Dendrobium formosum. Rev. Bras. Farmacogn. 2017;27:480–487. doi: 10.1016/j.bjp.2017.05.005. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources