Pulling the Brakes on Fast and Furious Multiple Drug-Resistant (MDR) Bacteria
- PMID: 33467089
- PMCID: PMC7830236
- DOI: 10.3390/ijms22020859
Pulling the Brakes on Fast and Furious Multiple Drug-Resistant (MDR) Bacteria
Abstract
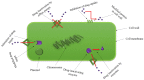
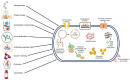
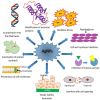
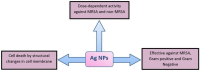
Life-threatening bacterial infections have been managed by antibiotics for years and have significantly improved the wellbeing and lifetime of humans. However, bacteria have always been one step ahead by inactivating the antimicrobial agent chemically or by producing certain enzymes. The alarming universal occurrence of multidrug-resistant (MDR) bacteria has compelled researchers to find alternative treatments for MDR infections. This is a menace where conventional chemotherapies are no longer promising, but several novel approaches could help. Our current review article discusses the novel approaches that can combat MDR bacteria: starting off with potential nanoparticles (NPs) that efficiently interact with microorganisms causing fatal changes in the morphology and structure of these cells; nanophotothermal therapy using inorganic NPs like AuNPs to destroy pathogenic bacterial cells; bacteriophage therapy against which bacteria develop less resistance; combination drugs that act on dissimilar targets in distinctive pathways; probiotics therapy by the secretion of antibacterial chemicals; blockage of quorum sensing signals stopping bacterial colonization, and vaccination against resistant bacterial strains along with virulence factors. All these techniques show us a promising future in the fight against MDR bacteria, which remains the greatest challenge in public health care.
Keywords: bacteriophages; combination therapy; multidrug resistance; nanoantibiotics; nanoparticles.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

