Possible Role of Metformin as an Immune Modulator in the Tumor Microenvironment of Ovarian Cancer
- PMID: 33467127
- PMCID: PMC7830067
- DOI: 10.3390/ijms22020867
Possible Role of Metformin as an Immune Modulator in the Tumor Microenvironment of Ovarian Cancer
Abstract
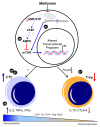
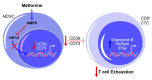
Growing evidence suggests that the immune component of the tumor microenvironment (TME) may be highly involved in the progression of high-grade serous ovarian cancer (HGSOC), as an immunosuppressive TME is associated with worse patient outcomes. Due to the poor prognosis of HGSOC, new therapeutic strategies targeting the TME may provide a potential path forward for preventing disease progression to improve patient survival. One such postulated approach is the repurposing of the type 2 diabetes medication, metformin, which has shown promise in reducing HGSOC tumor progression in retrospective epidemiological analyses and through numerous preclinical studies. Despite its potential utility in treating HGSOC, and that the immune TME is considered as a key factor in the disease's progression, little data has definitively shown the ability of metformin to target this component of the TME. In this brief review, we provide a summary of the current understanding of the effects of metformin on leukocyte function in ovarian cancer and, coupled with data from other related disease states, posit the potential mechanisms by which the drug may enhance the anti-tumorigenic effects of immune cells to improve HGSOC patient survival.
Keywords: T cell; macrophage; metformin; myeloid-derived suppressor cell; neutrophil; omentum; ovarian cancer; tumor microenvironment.
Conflict of interest statement
The authors declare that there are no competing interest.
Figures

References
-
- Doubeni C.A., Doubeni A.R., Myers A.E. Diagnosis and management of ovarian cancer. Am. Fam. Physician. 2016;93:937–944. - PubMed
-
- Surveillance, Epidemiology, and End Results (SEER) Program [(accessed on 10 November 2020)];Cancer Stat Facts: Ovarian Cancer. SEER* State Database: Mortality—All COD, Aggregated with State, Total, U.S. (1969–2017); Underlying Mortality Data Provided by NCHS. Available online: www.seer.cancer.gov/statfacts/html/ovary.html; www.cdc.gov/nchs.
-
- Winter W.E., Maxwell G.L., Tian C., Sundborg M.J., Rose G.S., Rose P.G., Rubin S.C., Muggia F.M., McGuire W.P. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage iv epithelial ovarian cancer: A gynecologic oncology group study. J. Clin. Oncol. 2008;26:83–89. doi: 10.1200/JCO.2007.13.1953. - DOI - PubMed
-
- Winter W.E., Maxwell G.L., Tian C., Carlson J.W., Ozols R.F., Rose P.G., Markman M., Armstrong D.K., Muggia F., McGuire W.P. Prognostic factors for stage iii epithelial ovarian cancer: A gynecologic oncology group study. J. Clin. Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

