Immunofluorescence Evaluation of Myf5 and MyoD in Masseter Muscle of Unilateral Posterior Crossbite Patients
- PMID: 33467295
- PMCID: PMC7739332
- DOI: 10.3390/jfmk5040080
Immunofluorescence Evaluation of Myf5 and MyoD in Masseter Muscle of Unilateral Posterior Crossbite Patients
Abstract
A unilateral posterior crossbite is a malocclusion where the low activity of the affected masseter muscle is compensated by the contralateral muscle hypertrophy. It is still unknown if, in the same condition, myogenesis with new fibre formation takes place.
Aim: the aim of the present study was to evaluate the expression of myogenesis markers, such as Myf5 and MyoD, in masseter muscles of unilateral posterior crossbite patients.
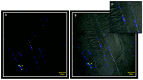
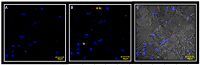
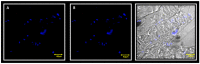
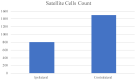
Materials and methods: biopsies from fifteen surgical patients with unilateral posterior crossbites have been analysed by immunofluorescence reactions. The results show the expression of Myf5 and MyoD in the contralateral muscle but not in the ipsilateral one. Moreover, statistical analysis shows the higher number of satellite cells in the contralateral side if compared to the ipsilateral one.
Conclusions: these results suggest that in contralateral muscle, hyperplastic events take place, as well as hypertrophy.
Keywords: Myf5; MyoD; PAX7; immunofluorescence; masseter muscle; unilateral posterior crossbite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Morphofunctional compensation of masseter muscles in unilateral posterior crossbite patients.Eur J Histochem. 2016 Jun 13;60(2):2605. doi: 10.4081/ejh.2016.2605. Eur J Histochem. 2016. PMID: 27349311 Free PMC article.
-
Masseter muscle thickness before and after the correction of unilateral functional posterior crossbite in growing individuals: a prospective controlled clinical trial.Eur J Orthod. 2024 Dec 4;47(1):cjae078. doi: 10.1093/ejo/cjae078. Eur J Orthod. 2024. PMID: 39729031 Clinical Trial.
-
Muscular activation during reverse and non-reverse chewing cycles in unilateral posterior crossbite.Eur J Oral Sci. 2009 Apr;117(2):122-8. doi: 10.1111/j.1600-0722.2008.00601.x. Eur J Oral Sci. 2009. PMID: 19320720
-
The molecular regulation of muscle stem cell function.Cold Spring Harb Symp Quant Biol. 2008;73:323-31. doi: 10.1101/sqb.2008.73.064. Epub 2009 Mar 27. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19329572 Review.
-
Exploring the potential of rapid maxillary expansion and masticatory muscle activity in unilateral posterior crossbite.J Clin Exp Dent. 2024 Jun 1;16(6):e755-e771. doi: 10.4317/jced.61604. eCollection 2024 Jun. J Clin Exp Dent. 2024. PMID: 39183996 Free PMC article. Review.
Cited by
-
Dystrophin-Glycoprotein Complex Behavior in Sternocleidomastoid Muscle of High- and Low-Ranking Baboons: A Possible Phylogenetic Arrangement.J Funct Morphol Kinesiol. 2022 Aug 25;7(3):62. doi: 10.3390/jfmk7030062. J Funct Morphol Kinesiol. 2022. PMID: 36135420 Free PMC article.
-
Histological and Immunohistochemical Insights into Disc Perforation in the Temporomandibular Joint: A Case Report.J Funct Morphol Kinesiol. 2025 Mar 27;10(2):107. doi: 10.3390/jfmk10020107. J Funct Morphol Kinesiol. 2025. PMID: 40566404 Free PMC article.
-
Temporomandibular Disorders Slow Down the Regeneration Process of Masticatory Muscles: Transcriptomic Analysis.Medicina (Kaunas). 2021 Apr 7;57(4):354. doi: 10.3390/medicina57040354. Medicina (Kaunas). 2021. PMID: 33916982 Free PMC article.

