Nanoporous Gold Monolith for High Loading of Unmodified Doxorubicin and Sustained Co-Release of Doxorubicin-Rapamycin
- PMID: 33467416
- PMCID: PMC7830488
- DOI: 10.3390/nano11010208
Nanoporous Gold Monolith for High Loading of Unmodified Doxorubicin and Sustained Co-Release of Doxorubicin-Rapamycin
Abstract
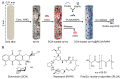
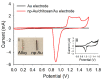
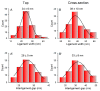
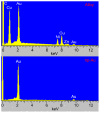
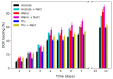
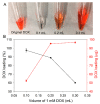
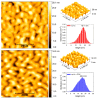
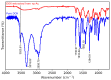
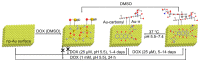
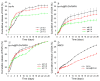
Nanoparticles (NPs) have been widely explored for delivering doxorubicin (DOX), an anticancer drug, to minimize cardiotoxicity. However, their efficiency is marred by a necessity to chemically modify DOX, NPs, or both and low deposition of the administered NPs on tumors. Therefore, alternative strategies should be developed to improve therapeutic efficacy and decrease toxicity. Here we report the possibility of employing a monolithic nanoporous gold (np-Au) rod as an implant for delivering DOX. The np-Au has very high DOX encapsulation efficiency (>98%) with maximum loading of 93.4 mg cm-3 without any chemical modification required of DOX or np-Au. We provide a plausible mechanism for the high loading of DOX in np-Au. The DOX sustained release for 26 days from np-Au in different pH conditions at 37 °C, which was monitored using UV-Vis spectroscopy. Additionally, we encased the DOX-loaded np-Au with rapamycin (RAPA)-trapped poly(D,L-lactide-co-glycolide) (PLGA) to fabricate an np-Au@PLGA/RAPA implant and optimized the combinatorial release of DOX and RAPA. Further exploiting the effect of the protein corona around np-Au and np-Au@PLGA/RAPA showed zero-order release kinetics of DOX. This work proves that the np-Au-based implant has the potential to be used as a DOX carrier of potential use in cancer treatment.
Keywords: doxorubicin; implant; nanoporous gold; rapamycin; sustained drug release.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
99mTc-doxorubicin-loaded gallic acid-gold nanoparticles (99mTc-DOX-loaded GA-Au NPs) as a multifunctional theranostic agent.Int J Pharm. 2020 Aug 30;586:119514. doi: 10.1016/j.ijpharm.2020.119514. Epub 2020 Jun 18. Int J Pharm. 2020. PMID: 32565281
-
Biodegradable poly(D,L-lactide-co-glycolide)/poly(L-γ-glutamic acid) nanoparticles conjugated to folic acid for targeted delivery of doxorubicin.Mater Sci Eng C Mater Biol Appl. 2017 Jul 1;76:743-751. doi: 10.1016/j.msec.2017.03.145. Epub 2017 Mar 18. Mater Sci Eng C Mater Biol Appl. 2017. PMID: 28482586
-
Incorporation and in vitro release of doxorubicin in thermally sensitive micelles made from poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide)-b-poly(D,L-lactide-co-glycolide) with varying compositions.Biomaterials. 2005 Aug;26(24):5064-74. doi: 10.1016/j.biomaterials.2005.01.030. Biomaterials. 2005. PMID: 15769542
-
Preparation and characterization of magnetic gold nanoparticles to be used as doxorubicin nanocarriers.Phys Med. 2014 Nov;30(7):843-8. doi: 10.1016/j.ejmp.2014.05.012. Epub 2014 Jun 18. Phys Med. 2014. PMID: 24950615
-
Interaction of gold nanoparticles with Doxorubicin mediated by supramolecular chemistry.Colloids Surf B Biointerfaces. 2015 Apr 1;128:237-244. doi: 10.1016/j.colsurfb.2015.01.041. Epub 2015 Feb 4. Colloids Surf B Biointerfaces. 2015. PMID: 25697809
Cited by
-
Applications of Nanoporous Gold in Therapy, Drug Delivery, and Diagnostics.Metals (Basel). 2023 Jan;13(1):78. doi: 10.3390/met13010078. Epub 2022 Dec 28. Metals (Basel). 2023. PMID: 39238564 Free PMC article.
-
Natural compounds-based nanomedicines for cancer treatment: Future directions and challenges.Drug Deliv Transl Res. 2024 Oct;14(10):2845-2916. doi: 10.1007/s13346-024-01649-z. Epub 2024 Jul 13. Drug Deliv Transl Res. 2024. PMID: 39003425 Free PMC article. Review.
-
Chemically-Gated and Sustained Molecular Transport through Nanoporous Gold Thin Films in Biofouling Conditions.Nanomaterials (Basel). 2021 Feb 16;11(2):498. doi: 10.3390/nano11020498. Nanomaterials (Basel). 2021. PMID: 33669404 Free PMC article.
-
Real-Time Monitoring of Doxorubicin Release from Hybrid Nanoporous Anodic Alumina Structures.Sensors (Basel). 2021 Nov 24;21(23):7819. doi: 10.3390/s21237819. Sensors (Basel). 2021. PMID: 34883823 Free PMC article.
-
p53 at the Crossroads between Doxorubicin-Induced Cardiotoxicity and Resistance: A Nutritional Balancing Act.Nutrients. 2023 May 10;15(10):2259. doi: 10.3390/nu15102259. Nutrients. 2023. PMID: 37242146 Free PMC article. Review.
References
-
- O’Brien M.E., Wigler N., Inbar M., Rosso R., Grischke E., Santoro A., Catane R., Kieback D., Tomczak P., Ackland S. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

