MRGPRX2 Activation by Rocuronium: Insights from Studies with Human Skin Mast Cells and Missense Variants
- PMID: 33467419
- PMCID: PMC7830812
- DOI: 10.3390/cells10010156
MRGPRX2 Activation by Rocuronium: Insights from Studies with Human Skin Mast Cells and Missense Variants
Abstract
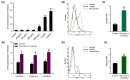
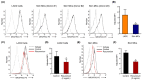
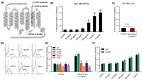
Perioperative hypersensitivity (POH) to the neuromuscular blocking drug (NMBD) rocuronium was previously thought to be IgE and mast cell (MC)-mediated. However, the recent seminal observation that rocuronium induces degranulation in murine peritoneal MCs (PMCs) via Mas-related G protein-coupled receptor B2 (MrgprB2) led to the idea that POH to this drug involves the activation of MRGPRX2 (human ortholog of MrgprB2). Furthermore, based on the demonstration that a patient with POH to rocuronium displayed three missense mutations (M196I, L226P and L237P) in MRGPRX2's transmembrane domains, it was proposed that this hypersensitivity reaction resulted from aberrant activation of this receptor. We found that rocuronium at 20 µg/mL caused degranulation in mouse PMCs via MrgprB2 but required at least 500 µg/mL to induce degranulation in human MCs via MRGPRX2. Furthermore, RBL-2H3 cells transiently expressing M196I, L226P and L237P variants did not display enhanced degranulation in response to rocuronium when compared to the wild-type receptor. These findings provide the first demonstration that rocuronium induces degranulation in human MCs via MRGPRX2. Furthermore, the important differences between MrgprB2 and MRGPRX2 and the inability of rocuronium to induce enhanced response in cells expressing MRGPRX2 variants suggest that the mechanism of its POH is more complex than previously thought.
Keywords: MRGPRX2; MrgprB2; anaphylaxis; mast cells; missense mutation; rocuronium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Van Gasse A.L., Elst J., Bridts C.H., Mertens C., Faber M., Hagendorens M.M., De Clerck L.S., Sabato V., Ebo D.G. Rocuronium Hypersensitivity: Does Off-Target Occupation of the MRGPRX2 Receptor Play a Role? J. Allergy Clin. Immunol. Pract. 2019;7:998–1003. doi: 10.1016/j.jaip.2018.09.034. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

