Molecular Delivery of Cytotoxic Agents via Integrin Activation
- PMID: 33467465
- PMCID: PMC7830197
- DOI: 10.3390/cancers13020299
Molecular Delivery of Cytotoxic Agents via Integrin Activation
Abstract
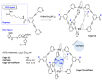
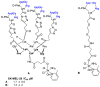
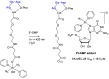
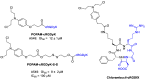
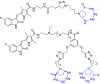
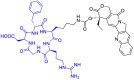
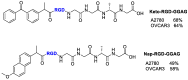
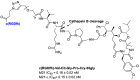
Integrins are cell adhesion receptors overexpressed in tumor cells. A direct inhibition of integrins was investigated, but the best inhibitors performed poorly in clinical trials. A gained attention towards these receptors arouse because they could be target for a selective transport of cytotoxic agents. Several active-targeting systems have been developed to use integrins as a selective cell entrance for some antitumor agents. The aim of this review paper is to report on the most recent results on covalent conjugates between integrin ligands and antitumor drugs. Cytotoxic drugs thus conjugated through specific linker to integrin ligands, mainly RGD peptides, demonstrated that the covalent conjugates were more selective against tumor cells and hopefully with fewer side effects than the free drugs.
Keywords: active targeting; cancer; integrins; molecular delivery; receptor targeting; selective citotoxycity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









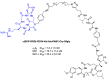



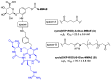

Similar articles
-
Integrin-Targeted Peptide- and Peptidomimetic-Drug Conjugates for the Treatment of Tumors.Recent Pat Anticancer Drug Discov. 2017;12(2):148-168. doi: 10.2174/1574892812666170203151930. Recent Pat Anticancer Drug Discov. 2017. PMID: 28164756 Review.
-
Integrin-targeted delivery into cancer cells of a Pt(IV) pro-drug through conjugation to RGD-containing peptides.Dalton Trans. 2015 Jan 7;44(1):202-12. doi: 10.1039/c4dt02710h. Dalton Trans. 2015. PMID: 25369773
-
The organometallic ferrocene exhibits amplified anti-tumor activity by targeted delivery via highly selective ligands to αvβ3, αvβ6, or α5β1 integrins.Biomaterials. 2021 Apr;271:120754. doi: 10.1016/j.biomaterials.2021.120754. Epub 2021 Mar 12. Biomaterials. 2021. PMID: 33756215
-
RGD-mediated delivery of small-molecule drugs.Future Med Chem. 2017 Apr;9(6):579-604. doi: 10.4155/fmc-2017-0008. Epub 2017 Apr 10. Future Med Chem. 2017. PMID: 28394627 Review.
-
Delivery of Theranostic Nanoparticles to Various Cancers by Means of Integrin-Binding Peptides.Int J Mol Sci. 2022 Nov 8;23(22):13735. doi: 10.3390/ijms232213735. Int J Mol Sci. 2022. PMID: 36430214 Free PMC article. Review.
Cited by
-
Interpreting the function of cell penetrating peptide (RGD) in drug transport to the cell membrane: a computational approach.Sci Rep. 2024 Nov 29;14(1):29668. doi: 10.1038/s41598-024-80060-7. Sci Rep. 2024. PMID: 39613819 Free PMC article.
-
Fabrication of curcumin-loaded magnetic PEGylated-PLGA nanocarriers tagged with GRGDS peptide for improving anticancer activity.MethodsX. 2023 May 23;10:102229. doi: 10.1016/j.mex.2023.102229. eCollection 2023. MethodsX. 2023. PMID: 37292239 Free PMC article.
-
Selective Integrin Ligands Promote Cell Internalization of the Antineoplastic Agent Fluorouracil.ACS Pharmacol Transl Sci. 2021 Sep 2;4(5):1528-1542. doi: 10.1021/acsptsci.1c00094. eCollection 2021 Oct 8. ACS Pharmacol Transl Sci. 2021. PMID: 34661072 Free PMC article.
-
Targeting Integrins for Cancer Therapy - Disappointments and Opportunities.Front Cell Dev Biol. 2022 Mar 9;10:863850. doi: 10.3389/fcell.2022.863850. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35356286 Free PMC article. Review.
-
Targeting Cytotoxic Agents through EGFR-Mediated Covalent Binding and Release.J Med Chem. 2023 Sep 14;66(17):12324-12341. doi: 10.1021/acs.jmedchem.3c00845. Epub 2023 Aug 30. J Med Chem. 2023. PMID: 37647129 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

