Non-Invasive Method to Detect Infection with Mycobacterium tuberculosis Complex in Wild Boar by Measurement of Volatile Organic Compounds Obtained from Feces with an Electronic Nose System
- PMID: 33467480
- PMCID: PMC7829825
- DOI: 10.3390/s21020584
Non-Invasive Method to Detect Infection with Mycobacterium tuberculosis Complex in Wild Boar by Measurement of Volatile Organic Compounds Obtained from Feces with an Electronic Nose System
Abstract
More effective methods to detect bovine tuberculosis, caused by Mycobacterium bovis, in wildlife, is of paramount importance for preventing disease spread to other wild animals, livestock, and human beings. In this study, we analyzed the volatile organic compounds emitted by fecal samples collected from free-ranging wild boar captured in Doñana National Park, Spain, with an electronic nose system based on organically-functionalized gold nanoparticles. The animals were separated by the age group for performing the analysis. Adult (>24 months) and sub-adult (12-24 months) animals were anesthetized before sample collection, whereas the juvenile (<12 months) animals were manually restrained while collecting the sample. Good accuracy was obtained for the adult and sub-adult classification models: 100% during the training phase and 88.9% during the testing phase for the adult animals, and 100% during both the training and testing phase for the sub-adult animals, respectively. The results obtained could be important for the further development of a non-invasive and less expensive detection method of bovine tuberculosis in wildlife populations.
Keywords: Mycobacterium bovis; Sus scrofa; bovine tuberculosis; chemical gas sensors; diagnosis; feces; gold nanoparticles; organic ligands; volatile organic compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


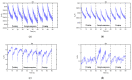
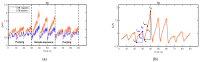

References
-
- Di Marco V., Mazzone P., Capucchio M.T., Boniotti M.B., Aronica V., Russo M., Fiasconaro M., Cifani N., Corneli S., Biasibetti E., et al. Epidemiological significance of the domestic black pig (Sus scrofa) in maintenance of bovine tuberculosis in Sicily. J. Clin. Microbiol. 2012;50:1209–1218. doi: 10.1128/JCM.06544-11. - DOI - PMC - PubMed
-
- Bapat P.R., Dodkey R.S., Shekhawat S.D., Husain A.A., Nayak A.R., Kawle A.P., Daginawala H.F., Singh L.K., Kashyap R.S. Prevalence of zoonotic tuberculosis and associated risk factors in Central Indian populations. J. Epidemiol. Glob. Health. 2017;7:277–283. doi: 10.1016/j.jegh.2017.08.007. - DOI - PMC - PubMed
-
- Richeldi L. Rapid identification of Mycobacterium tuberculosis infection. Clin. Microbiol. Infect. 2006;12:34–36. doi: 10.1111/j.1469-0691.2006.01655.x. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

