Differential Bacterial Predation by Free-Living Amoebae May Result in Blooms of Legionella in Drinking Water Systems
- PMID: 33467483
- PMCID: PMC7829821
- DOI: 10.3390/microorganisms9010174
Differential Bacterial Predation by Free-Living Amoebae May Result in Blooms of Legionella in Drinking Water Systems
Abstract
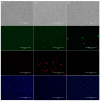
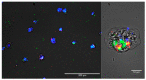
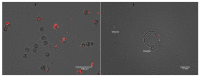
Intracellular growth of pathogenic Legionella in free-living amoebae (FLA) results in the critical concentrations that are problematic in engineered water systems (EWS). However, being amoeba-resistant bacteria (ARB), how Legionella spp. becomes internalized within FLA is still poorly understood. Using fluorescent microscopy, we investigated in real-time the preferential feeding behavior of three water-related FLA species, Willaertia magna, Acanthamoeba polyphaga, and Vermamoeba vermiformis regarding Legionella pneumophila and two Escherichia coli strains. Although all the studied FLA species supported intracellular growth of L. pneumophila, they avoided this bacterium to a certain degree in the presence of E. coli and mostly fed on it when the preferred bacterial food-sources were limited. Moreover, once L. pneumophila were intracellular, it inhibited digestion of co-occurring E. coli within the same trophozoites. Altogether, based on FLA-bacteria interactions and the shifts in microbial population dynamics, we propose that FLA's feeding preference leads to an initial growth of FLA and depletion of prey bacteria, thus increases the relative abundance of Legionella and creates a "forced-feeding" condition facilitating the internalization of Legionella into FLA to initiate the cycles of intracellular multiplication. These findings imply that monitoring of FLA levels in EWS could be useful in predicting possible imminent high occurrence of Legionella.
Keywords: Legionella; Legionnaires’ disease; engineered water systems; free-living amoebae.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Long-term persistence of infectious Legionella with free-living amoebae in drinking water biofilms.Int J Hyg Environ Health. 2019 May;222(4):678-686. doi: 10.1016/j.ijheh.2019.04.007. Epub 2019 Apr 27. Int J Hyg Environ Health. 2019. PMID: 31036480
-
Diversity of Free-Living Amoebae in New Zealand Groundwater and Their Ability to Feed on Legionella pneumophila.Pathogens. 2024 Aug 7;13(8):665. doi: 10.3390/pathogens13080665. Pathogens. 2024. PMID: 39204265 Free PMC article.
-
Characterization of the natural bacterial microbiota of pathogenic free-living amoebae (Acanthamoeba spp. and Naegleria fowleri) isolated from rivers and tap water in Guadeloupe.Sci Total Environ. 2025 May 1;975:179204. doi: 10.1016/j.scitotenv.2025.179204. Epub 2025 Apr 5. Sci Total Environ. 2025. PMID: 40187337
-
Ecology of free-living amoebae.Crit Rev Microbiol. 1994;20(3):225-41. doi: 10.3109/10408419409114556. Crit Rev Microbiol. 1994. PMID: 7802958 Review.
-
Free-living amoebae protecting Legionella in water: the tip of an iceberg?Scand J Infect Dis. 1999;31(4):383-5. doi: 10.1080/00365549950163833. Scand J Infect Dis. 1999. PMID: 10528878 Review.
Cited by
-
Tenets of a holistic approach to drinking water-associated pathogen research, management, and communication.Water Res. 2022 Mar 1;211:117997. doi: 10.1016/j.watres.2021.117997. Epub 2021 Dec 22. Water Res. 2022. PMID: 34999316 Free PMC article. Review.
-
Legionella pneumophila response to shifts in biofilm structure mediated by hydrodynamics.Biofilm. 2025 Jan 24;9:100258. doi: 10.1016/j.bioflm.2025.100258. eCollection 2025 Jun. Biofilm. 2025. PMID: 39957834 Free PMC article.
-
Use of a Novel DNA-Loaded Alginate-Calcium Carbonate Biopolymer Surrogate to Study the Engulfment of Legionella pneumophila by Acanthamoeba polyphaga in Water Systems.Microbiol Spectr. 2022 Aug 31;10(4):e0221022. doi: 10.1128/spectrum.02210-22. Epub 2022 Aug 11. Microbiol Spectr. 2022. PMID: 35950853 Free PMC article.
-
Foresight 2035: a perspective on the next decade of research on the management of Legionella spp. in engineered aquatic environments.FEMS Microbiol Rev. 2025 Jan 14;49:fuaf022. doi: 10.1093/femsre/fuaf022. FEMS Microbiol Rev. 2025. PMID: 40424003 Free PMC article. Review.
-
Quantitative Microbial Risk Assessment Framework Incorporating Water Ages with Legionella pneumophila Growth Rates.Environ Sci Technol. 2024 Apr 16;58(15):6540-6551. doi: 10.1021/acs.est.4c01208. Epub 2024 Apr 4. Environ Sci Technol. 2024. PMID: 38574283 Free PMC article.
References
-
- Beer K.D., Gargano J.W., Roberts V.A., Hill V.R., Garrison L.E., Kutty P.K., Hilborn E.D., Wade T.J., Fullerton K.E., Yoder J.S. Surveillance for Waterborne Disease Outbreaks Associated with Drinking Water—United States, 2011–2012. Morb. Mortal. Wkly. Rep. 2015;64:842–848. doi: 10.15585/mmwr.mm6431a2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

