Additive-Manufactured Gyroid Scaffolds of Magnesium Oxide, Phosphate Glass Fiber and Polylactic Acid Composite for Bone Tissue Engineering
- PMID: 33467495
- PMCID: PMC7830155
- DOI: 10.3390/polym13020270
Additive-Manufactured Gyroid Scaffolds of Magnesium Oxide, Phosphate Glass Fiber and Polylactic Acid Composite for Bone Tissue Engineering
Abstract
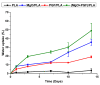
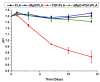
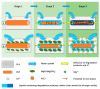
Composites of biodegradable phosphate glass fiber and polylactic acid (PGF/PLA) show potential for bone tissue engineering scaffolds, due to their ability to release Ca, P, and Mg during degradation, thus promoting the bone repair. Nevertheless, glass degradation tends to acidify the surrounding aqueous environment, which may adversely affect the viability and bone-forming activities of osteoblasts. In this work, MgO was investigated as a neutralizing agent. Porous network-phase gyroid scaffolds were additive-manufactured using four different materials: PLA, MgO/PLA, PGF/PLA, and (MgO + PGF)/PLA. The addition of PGF enhanced compressive properties of scaffolds, and the resultant scaffolds were comparably strong and stiff with human trabecular bone. While the degradation of PGF/PLA composite induced considerable acidity in degradation media and intensified the degradation of PGF in return, the degradation media of (MgO + PGF)/PLA maintained a neutral pH close to a physiological environment. The experiment results indicated the possible mechanism of MgO as the neutralizing agent: the local acidity was buffered as the MgO reacted with the acidic degradation products thereby inhibiting the degradation of PGF from being intensified in an acidic environment. The (MgO + PGF)/PLA composite scaffold appears to be a candidate for bone tissue engineering.
Keywords: additive manufacturing; bone tissue engineering scaffold; gyroid; phosphate glass fiber; polylactic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Williams D., Zhang X., editors. Definitions of Biomaterials for the Twenty-First Century. Elsevier; Amsterdam, The Netherlands: 2019. VI—Regenerative medicine; pp. 115–153. - DOI
-
- Bose S., Vahabzadeh S., Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater. Today. 2013;16:496–504. doi: 10.1016/j.mattod.2013.11.017. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

