Crosstalk of the Caspase Family and Mammalian Target of Rapamycin Signaling
- PMID: 33467535
- PMCID: PMC7830632
- DOI: 10.3390/ijms22020817
Crosstalk of the Caspase Family and Mammalian Target of Rapamycin Signaling
Abstract
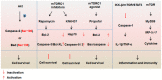
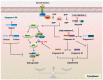
Cell can integrate the caspase family and mammalian target of rapamycin (mTOR) signaling in response to cellular stress triggered by environment. It is necessary here to elucidate the direct response and interaction mechanism between the two signaling pathways in regulating cell survival and determining cell fate under cellular stress. Members of the caspase family are crucial regulators of inflammation, endoplasmic reticulum stress response and apoptosis. mTOR signaling is known to mediate cell growth, nutrition and metabolism. For instance, over-nutrition can cause the hyperactivation of mTOR signaling, which is associated with diabetes. Nutrition deprivation can inhibit mTOR signaling via SH3 domain-binding protein 4. It is striking that Ras GTPase-activating protein 1 is found to mediate cell survival in a caspase-dependent manner against increasing cellular stress, which describes a new model of apoptosis. The components of mTOR signaling-raptor can be cleaved by caspases to control cell growth. In addition, mTOR is identified to coordinate the defense process of the immune system by suppressing the vitality of caspase-1 or regulating other interferon regulatory factors. The present review discusses the roles of the caspase family or mTOR pathway against cellular stress and generalizes their interplay mechanism in cell fate determination.
Keywords: cell fate; interplay; mTOR signaling; the caspase family.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling.Trends Cell Biol. 2012 May;22(5):274-82. doi: 10.1016/j.tcb.2012.02.006. Epub 2012 Mar 21. Trends Cell Biol. 2012. PMID: 22444729 Review.
-
Melatonin alleviates endoplasmic reticulum stress and follicular granulosa cell apoptosis by regulating ATF4 to activate mTOR signaling pathway in chickens.Poult Sci. 2024 Jun;103(6):103656. doi: 10.1016/j.psj.2024.103656. Epub 2024 Mar 15. Poult Sci. 2024. PMID: 38583308 Free PMC article.
-
GADD34 Keeps the mTOR Pathway Inactivated in Endoplasmic Reticulum Stress Related Autophagy.PLoS One. 2016 Dec 16;11(12):e0168359. doi: 10.1371/journal.pone.0168359. eCollection 2016. PLoS One. 2016. PMID: 27992581 Free PMC article.
-
mTOR pathway mediates endoplasmic reticulum stress-induced CD4+ T cell apoptosis in septic mice.Apoptosis. 2022 Oct;27(9-10):740-750. doi: 10.1007/s10495-022-01740-1. Epub 2022 Jun 27. Apoptosis. 2022. PMID: 35759162 Free PMC article.
-
Mammalian target of rapamycin (mTOR) as a potential therapeutic target in various diseases.Inflammopharmacology. 2017 Jun;25(3):293-312. doi: 10.1007/s10787-017-0336-1. Epub 2017 Apr 17. Inflammopharmacology. 2017. PMID: 28417246 Review.
Cited by
-
Induction of Apoptosis by Metabolites of Rhei Radix et Rhizoma (Da Huang): A Review of the Potential Mechanism in Hepatocellular Carcinoma.Front Pharmacol. 2022 Mar 2;13:806175. doi: 10.3389/fphar.2022.806175. eCollection 2022. Front Pharmacol. 2022. PMID: 35308206 Free PMC article. Review.
-
Inhibition of the Protein Arginine Methyltransferase PRMT5 in High-Risk Multiple Myeloma as a Novel Treatment Approach.Front Cell Dev Biol. 2022 Jun 8;10:879057. doi: 10.3389/fcell.2022.879057. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35757005 Free PMC article.
-
Based on Bioinformatics to Explore the Mechanism of "Tangzhiqing" Decoction Alleviating Type 2 Diabetes-associated Cognitive Dysfunction in Mice by Regulating Hippocampal Neuron Apoptosis and Autophagy.Comb Chem High Throughput Screen. 2024;27(17):2565-2582. doi: 10.2174/0113862073255849231030114405. Comb Chem High Throughput Screen. 2024. PMID: 37990900
-
Stimulation of α7-nAChRs coordinates autophagy and apoptosis signaling in experimental knee osteoarthritis.Cell Death Dis. 2021 May 5;12(5):448. doi: 10.1038/s41419-021-03726-4. Cell Death Dis. 2021. PMID: 33953172 Free PMC article.
-
Therapeutic Effects of (+)-Afzelechin on Particulate Matter-Induced Pulmonary Injury.Biomol Ther (Seoul). 2024 Jan 1;32(1):162-169. doi: 10.4062/biomolther.2023.187. Biomol Ther (Seoul). 2024. PMID: 38148560 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

