A Step-by-Step Approach to Improve Clinical Translation of Liposome-Based Nanomaterials, a Focus on Innate Immune and Inflammatory Responses
- PMID: 33467541
- PMCID: PMC7830677
- DOI: 10.3390/ijms22020820
A Step-by-Step Approach to Improve Clinical Translation of Liposome-Based Nanomaterials, a Focus on Innate Immune and Inflammatory Responses
Abstract
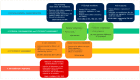
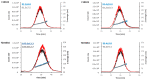
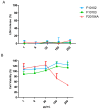
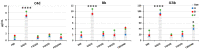
This study aims to provide guidelines to design and perform a robust and reliable physical-chemical characterization of liposome-based nanomaterials, and to support method development with a specific focus on their inflammation-inducing potential. Out of eight differently functionalized liposomes selected as "case-studies", three passed the physical-chemical characterization ( in terms of size-distribution, homogeneity and stability) and the screening for bacterial contamination (sterility and apyrogenicity). Although all three were non-cytotoxic when tested in vitro, they showed a different capacity to activate human blood cells. HSPC/CHOL-coated liposomes elicited the production of several inflammation-related cytokines, while DPPC/CHOL- or DSPC/CHOL-functionalized liposomes did not. This work underlines the need for accurate characterization at multiple levels and the use of reliable in vitro methods, in order to obtain a realistic assessment of liposome-induced human inflammatory response, as a fundamental requirement of nanosafety regulations.
Keywords: Limulus Amoebocyte Lisate (LAL); cytokines; endotoxin; inflammation; interleukin; liposome; nanomaterial; nanomedicine; particle size distribution; physicochemical characterization; safety assessment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- FDA News Release FDA Approves First Treatment for Certain Types of Poor-Prognosis Acute Myeloid Leukemia. [(accessed on 9 December 2020)];2017 Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-t....
-
- Krauss A.C., Gao X., Li L., Manning M.L., Patel P., Fu W., Janoria K.G., Gieser G., Bateman D.A., Przepiorka D., et al. FDA Approval Summary: (Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid Leukemia. Clin. Cancer Res. 2019;25:2685–2690. doi: 10.1158/1078-0432.CCR-18-2990. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

