Canadian Consensus for Biomarker Testing and Treatment of TRK Fusion Cancer in Adults
- PMID: 33467570
- PMCID: PMC7903287
- DOI: 10.3390/curroncol28010053
Canadian Consensus for Biomarker Testing and Treatment of TRK Fusion Cancer in Adults
Abstract
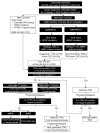
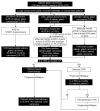
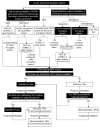
The tyrosine receptor kinase (TRK) inhibitors larotrectinib and entrectinib were recently approved in Canada for the treatment of solid tumours harbouring neurotrophic tyrosine receptor kinase (NTRK) gene fusions. These NTRK gene fusions are oncogenic drivers found in most tumour types at a low frequency (<5%), and at a higher frequency (>80%) in a small number of rare tumours (e.g., secretory carcinoma of the salivary gland and of the breast). They are generally mutually exclusive of other common oncogenic drivers. Larotrectinib and entrectinib have demonstrated impressive overall response rates and tolerability in Phase I/II trials in patients with TRK fusion cancer with no other effective treatment options. Given the low frequency of TRK fusion cancer and the heterogeneous molecular testing landscape in Canada, identifying and optimally managing such patients represents a new challenge. We provide a Canadian consensus on when and how to test for NTRK gene fusions and when to consider treatment with a TRK inhibitor. We focus on five tumour types: thyroid carcinoma, colorectal carcinoma, non-small cell lung carcinoma, soft tissue sarcoma, and salivary gland carcinoma. Based on the probability of the tumour harbouring an NTRK gene fusion, we also suggest a tumour-agnostic consensus for NTRK gene fusion testing and treatment. We recommend considering a TRK inhibitor in all patients with TRK fusion cancer with no other effective treatment options.
Trial registration: ClinicalTrials.gov NCT02122913 NCT02637687 NCT02576431.
Keywords: NTRK; TRK fusion; entrectinib; larotrectinib; molecular testing; oncogenic drivers; targeted therapy; tumour-agnostic.
Conflict of interest statement
We have read and understood Current Oncology’s policy on conflicts of interest disclosure and declare the following interests: D.G.B.: Advisory Boards: A.Z., B.I., Lilly, B.M.S., Merck, Amgen, Pfizer, Novartis, Roche, Bayer; Research Funding: A.Z., B.I., Pfizer; Meeting Sponsorships: POET Annual Meeting: Takeda, A.Z., B.I., Lilly, B.M.S., Merck, Pfizer, Novartis, Roche, Bayer, Illumina, Glans-Look Lung Cancer Research Day: A.Z., B.I., Roche. Ownership and Director, Medical Affairs: OncoHelix. S.B.: Advisory Board/Honorarium: AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers-Squibb, GlaxoSmithKline, Lilly, Merck, Novartis, Pfizer, Roche, Takeda. N.B.: Nothing to disclose. P.D.: Consultant (Astra-Zeneca, Eli Lilly); Presentations (Pfizer, Astra-Zeneca); Research funding (Pfizer, Novartis, Roche). S.G.: Advisory Boards and Speaking honoraria: Bayer, Amgen, Roche, Pfizer. A.G.: Nothing to disclose. H.F.: has received honoraria for either advisory boards or speaking engagements from EMD Serono, Pfizer, Bayer, Amgen, Astellas, Merck, Roche and Astra Zeneca; has current research and clinical lab support from EMD Serono and Astra Zeneca, past research support from Thermo Fisher, and her clinical lab has a contract arrangement with Bayer. A.R.H.: institutional support for clinical trials conduct from Novartis, Bristol-Myers Squibb, Pfizer, Boerhinger-Ingelheim, GlaxoSmithKline, Roche/Genentech, Karyopharm, AstraZeneca/ Medimmune, Merck, Astellas and Bayer and compensated consulting/advisory boards for AstraZeneca, Merck and GlaxoSmithKline. M.H.: Advisory Board Honoraria: Bayer, Merck, Roche. M.K.: Honoraria: Novartis, Eisai, Bayer; Consulting or Advisory Role: Eisai; Research Funding: Eisai (Inst), Exelixis (Inst), Ipsen (Inst). B.M.: Advisory Board/Honorarium: Bayer, Pfizer, Astra Zeneca, Boehringer Ingleheim, Roche. J.N.: Nothing to disclose. B.P.: I have received stipends from Bayer. D.R.: Nothing to disclose. C.S.: Nothing to disclose. D.S.: Ad boards participation with Bayer. E.T.: Torlakovic reports grants and other from BMS, grants from Pfizer, grants and other from Merck, grants and other from Roche, grants and other from AstraZeneca, outside the submitted work. M.S.T.: Honoraria: Bayer, Hoffmann La Roche; Research Grant: Bayer.
Figures



References
-
- Vaishnavi A., Le A.T., Doebele R.C. TRKing Down an Old Oncogene in a New Era of Targeted Therapy. Cancer Discov. 2015;5:25–34. doi: 10.1158/2159-8290.CD-14-0765. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

