Carbon Dots: An Emerging Smart Material for Analytical Applications
- PMID: 33467583
- PMCID: PMC7829846
- DOI: 10.3390/mi12010084
Carbon Dots: An Emerging Smart Material for Analytical Applications
Abstract
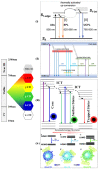
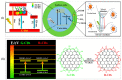
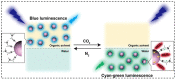
Carbon dots (CDs) are optically active carbon-based nanomaterials. These nanomaterials can change their light emission properties in response to various external stimuli such as pH, temperature, pressure, and light. The CD's remarkable stimuli-responsive smart material properties have recently stimulated massive research interest for their exploitation to develop various sensor platforms. Herein, an effort has been made to review the major advances made on CDs, focusing mainly on its smart material attributes and linked applications. Since the CD's material properties are largely linked to their synthesis approaches, various synthesis methods, including surface passivation and functionalization of CDs and the mechanisms reported so far in their photophysical properties, are also delineated in this review. Finally, the challenges of using CDs and the scope for their further improvement as an optical signal transducer to expand their application horizon for developing analytical platforms have been discussed.
Keywords: analytical; carbon dots; chemiluminescence; electrochemiluminescence; optical; photoluminescence; smart materials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Devi P., Rajput P., Thakur A., Kim K.-H., Kumar P. Recent Advances in Carbon Quantum Dot-Based Sensing of Heavy Metals in Water. TrAC Trends Anal. Chem. 2019;114:171–195. doi: 10.1016/j.trac.2019.03.003. - DOI
-
- Das S., Goswami P. Nanozymes as Potential Catalysts for Sensing and Analytical Applications. In: Goswami P., editor. Advanced Materials and Techniques for Biosensors and Bioanalytical Applications. 1st ed. CRC Press; Boca Raton, FL, USA: 2020. pp. 143–162.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

