Performance of a Four-Antigen Staphylococcus aureus Vaccine in Preclinical Models of Invasive S. aureus Disease
- PMID: 33467609
- PMCID: PMC7830931
- DOI: 10.3390/microorganisms9010177
Performance of a Four-Antigen Staphylococcus aureus Vaccine in Preclinical Models of Invasive S. aureus Disease
Abstract
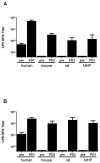
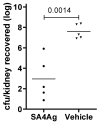
A Staphylococcus aureus four-antigen vaccine (SA4Ag) was designed for the prevention of invasive disease in surgical patients. The vaccine is composed of capsular polysaccharide type 5 and type 8 CRM197 conjugates, a clumping factor A mutant (Y338A-ClfA) and manganese transporter subunit C (MntC). S. aureus pathogenicity is characterized by an ability to rapidly adapt to the host environment during infection, which can progress from a local infection to sepsis and invasion of distant organs. To test the protective capacity of the SA4Ag vaccine against progressive disease stages of an invasive S. aureus infection, a deep tissue infection mouse model, a bacteremia mouse model, a pyelonephritis model, and a rat model of infectious endocarditis were utilized. SA4Ag vaccination significantly reduced the bacterial burden in deep tissue infection, in bacteremia, and in the pyelonephritis model. Complete prevention of infection was demonstrated in a clinically relevant endocarditis model. Unfortunately, these positive preclinical findings with SA4Ag did not prove the clinical utility of SA4Ag in the prevention of surgery-associated invasive S. aureus infection.
Keywords: ClfA; MntC; SA4Ag vaccine; Staphylococcus aureus; animal models; conjugated polysaccharide; invasive disease; protection; sepsis; surgery-associated infection.
Conflict of interest statement
I.L.S., Y.T., A.I., P.A.L., K.U.J. and A.S.A. are employees of Pfizer Inc. and may hold stock or stock options. P.L. was an employee of Pfizer at the time the work was done.
Figures

References
-
- Noskin G.A., Rubin R.J., Schentag J.J., Kluytmans J., Hedblom E.C., Jacobson C., Smulders M., Gemmen E., Bharmal M. National trends in Staphylococcus aureus infection rates: Impact on economic burden and mortality over a 6-year period (1998–2003) Clin. Infect. Dis. 2007;45:1132–1140. doi: 10.1086/522186. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

