Real Time PCR and Culture-Based Virus Isolation Test in Clinically Recovered Patients: Is the Subject Still Infectious for SARS-CoV2?
- PMID: 33467628
- PMCID: PMC7829794
- DOI: 10.3390/jcm10020309
Real Time PCR and Culture-Based Virus Isolation Test in Clinically Recovered Patients: Is the Subject Still Infectious for SARS-CoV2?
Abstract
Background: The highly variable manifestation of the COVID-19 disease, from completely asymptomatic to fatal, is both a clinical and a public health issue. The criteria for discharge of hospitalized patients have been based so far on the negative result of Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) tests, but the persistence of viral fragments may exceed that of the integral virus by weeks. The aim of our study was to verify the clearance of the virus at viral culture in patients hospitalized for COVID-19 that have clinically recovered but are still positive on nasopharyngeal swab.
Methods: The study was conducted in hospitalized patients with positive RT-PCR on nasopharyngeal swab. Patients included were from asymptomatic to severe cases and performed nasopharyngeal control swabbing on day 14 for asymptomatic patient or at least three days after remission of symptoms. RT-PCR positive specimens were sent to a biosafety level 3 laboratory for viral culture.
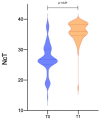
Results: We performed a combined analysis of RT-PCR and a highly sensitive in vitro culture from 84 samples of hospitalized patients. The average age was 46 ± 20.29, and 40.5% of the subjects had radiologically confirmed pneumonia, with average PaO2 of 72.35 ± 12.12and P/F ratio of 315 ± 83.15. Ct values for the N gene were lower in the first swab than in the control one (p < 0.001). The samples from 83 patients were negative at viral culture, and RT-PCR on the respective supernatants always confirmed the absence of viral growth.
Conclusions: Our preliminary results demonstrate that patients clinically recovered for at least three days show the viral clearance at viral culture, and presumably they continued to not be contagious.
Keywords: COVID-19; RT-PCR; SARS-CoV-2; cell culture; viral transmission.
Conflict of interest statement
All authors have not any conflict of interest to declare.
Figures

Similar articles
-
Assessing Viral Shedding and Infectivity of Asymptomatic or Mildly Symptomatic Patients with COVID-19 in a Later Phase.J Clin Med. 2020 Sep 10;9(9):2924. doi: 10.3390/jcm9092924. J Clin Med. 2020. PMID: 32927798 Free PMC article.
-
Detection and analysis of nucleic acid in various biological samples of COVID-19 patients.Travel Med Infect Dis. 2020 Sep-Oct;37:101673. doi: 10.1016/j.tmaid.2020.101673. Epub 2020 Apr 18. Travel Med Infect Dis. 2020. PMID: 32311437 Free PMC article.
-
Necessity for detection of SARS-CoV-2 RNA in multiple types of specimens for the discharge of the patients with COVID-19.J Transl Med. 2020 Nov 2;18(1):411. doi: 10.1186/s12967-020-02580-w. J Transl Med. 2020. PMID: 33138834 Free PMC article.
-
[SARS-CoV-2 and Microbiological Diagnostic Dynamics in COVID-19 Pandemic].Mikrobiyol Bul. 2020 Jul;54(3):497-509. doi: 10.5578/mb.69839. Mikrobiyol Bul. 2020. PMID: 32755524 Review. Turkish.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. PMID: 32997361 Updated.
Cited by
-
The reliability of saliva for the detection of SARS-CoV-2 in symptomatic and asymptomatic patients: Insights on the diagnostic performance and utility for COVID-19 screening.Diagn Microbiol Infect Dis. 2021 Nov;101(3):115450. doi: 10.1016/j.diagmicrobio.2021.115450. Epub 2021 Jun 6. Diagn Microbiol Infect Dis. 2021. PMID: 34284319 Free PMC article.
-
VOC 202012/01 Variant Is Effectively Neutralized by Antibodies Produced by Patients Infected before Its Diffusion in Italy.Viruses. 2021 Feb 11;13(2):276. doi: 10.3390/v13020276. Viruses. 2021. PMID: 33670182 Free PMC article.
-
The impacts of vaccination status and host factors during early infection on SARS-CoV-2 persistence:a retrospective single-center cohort study.Int Immunopharmacol. 2023 Jan;114:109534. doi: 10.1016/j.intimp.2022.109534. Epub 2022 Nov 30. Int Immunopharmacol. 2023. PMID: 36476489 Free PMC article.
-
Molecular Virology of SARS-CoV-2 and Related Coronaviruses.Microbiol Mol Biol Rev. 2022 Jun 15;86(2):e0002621. doi: 10.1128/mmbr.00026-21. Epub 2022 Mar 28. Microbiol Mol Biol Rev. 2022. PMID: 35343760 Free PMC article. Review.
-
Microbiological screening tests for SARS-CoV-2 in the first hour since the hospital admission: A reliable tool for enhancing the safety of pediatric care.Front Pediatr. 2022 Sep 6;10:966901. doi: 10.3389/fped.2022.966901. eCollection 2022. Front Pediatr. 2022. PMID: 36147810 Free PMC article.
References
-
- Covid-19—Situazione in Italia. [(accessed on 24 October 2020)]; Available online: http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuov....
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

