Current Advances in 3D Tissue and Organ Reconstruction
- PMID: 33467648
- PMCID: PMC7830719
- DOI: 10.3390/ijms22020830
Current Advances in 3D Tissue and Organ Reconstruction
Abstract
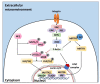
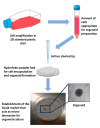
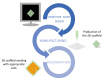
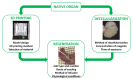
Bi-dimensional culture systems have represented the most used method to study cell biology outside the body for over a century. Although they convey useful information, such systems may lose tissue-specific architecture, biomechanical effectors, and biochemical cues deriving from the native extracellular matrix, with significant alterations in several cellular functions and processes. Notably, the introduction of three-dimensional (3D) platforms that are able to re-create in vitro the structures of the native tissue, have overcome some of these issues, since they better mimic the in vivo milieu and reduce the gap between the cell culture ambient and the tissue environment. 3D culture systems are currently used in a broad range of studies, from cancer and stem cell biology, to drug testing and discovery. Here, we describe the mechanisms used by cells to perceive and respond to biomechanical cues and the main signaling pathways involved. We provide an overall perspective of the most recent 3D technologies. Given the breadth of the subject, we concentrate on the use of hydrogels, bioreactors, 3D printing and bioprinting, nanofiber-based scaffolds, and preparation of a decellularized bio-matrix. In addition, we report the possibility to combine the use of 3D cultures with functionalized nanoparticles to obtain highly predictive in vitro models for use in the nanomedicine field.
Keywords: 3D matrices; 3D printing and bioprinting; biomechanical cues; decellularization; hydrogel; micro-bioreactor; microenvironment remodeling; nanofiber-based scaffolds; nanomedicine; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Harrison R.G., Greenman M.J., Mall F.P., Jackson C.M. Observations of the living developing nerve fiber. Anat. Rec. 1907;1:116–128. doi: 10.1002/ar.1090010503. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

