Optimized 5-Fluorouridine Prodrug for Co-Loading with Doxorubicin in Clinically Relevant Liposomes
- PMID: 33467652
- PMCID: PMC7830726
- DOI: 10.3390/pharmaceutics13010107
Optimized 5-Fluorouridine Prodrug for Co-Loading with Doxorubicin in Clinically Relevant Liposomes
Abstract
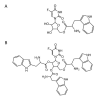
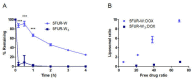
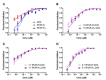
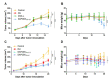
Liposome-based drug delivery systems have allowed for better drug tolerability and longer circulation times but are often optimized for a single agent due to the inherent difficulty of co-encapsulating two drugs with differing chemical profiles. Here, we design and test a prodrug based on a ribosylated nucleoside form of 5-fluorouracil, 5-fluorouridine (5FUR), with the final purpose of co-encapsulation with doxorubicin (DOX) in liposomes. To improve the loading of 5FUR, we developed two 5FUR prodrugs that involved the conjugation of either one or three moieties of tryptophan (W) known respectively as, 5FUR-W and 5FUR-W3. 5FUR-W demonstrated greater chemical stability than 5FUR-W3 and allowed for improved loading with fewer possible byproducts from tryptophan hydrolysis. Varied drug ratios of 5FUR-W: DOX were encapsulated for in vivo testing in the highly aggressive 4T1 murine breast cancer model. A liposomal molar ratio of 2.5 5FUR-W: DOX achieved a 62.6% reduction in tumor size compared to the untreated control group and a 33% reduction compared to clinical doxorubicin liposomes in a proof-of-concept study to demonstrate the viability of the co-encapsulated liposomes. We believe that the new prodrug 5FUR-W demonstrates a prodrug design with clinical translatability by reducing the number of byproducts produced by the hydrolysis of tryptophan, while also allowing for loading flexibility.
Keywords: drug combination; liposome; nanoparticle; targeting.
Conflict of interest statement
S.M. and M.B. are inventors on a patent application that related some aspects of the formulations described in this study (owned and managed by University of California). Promius Pharma had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Wang R., Billone P.S., Mullett W.M. Nanomedicine in Action: An Overview of Cancer Nanomedicine on the Market and in Clinical Trials. J. Nanomater. 2013;2013:629681. doi: 10.1155/2013/629681. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

