A Review of Graphene-Based Surface Plasmon Resonance and Surface-Enhanced Raman Scattering Biosensors: Current Status and Future Prospects
- PMID: 33467669
- PMCID: PMC7830205
- DOI: 10.3390/nano11010216
A Review of Graphene-Based Surface Plasmon Resonance and Surface-Enhanced Raman Scattering Biosensors: Current Status and Future Prospects
Abstract
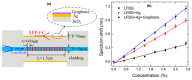
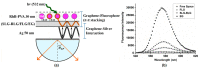
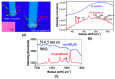
The surface plasmon resonance (SPR) biosensor has become a powerful analytical tool for investigating biomolecular interactions. There are several methods to excite surface plasmon, such as coupling with prisms, fiber optics, grating, nanoparticles, etc. The challenge in developing this type of biosensor is to increase its sensitivity. In relation to this, graphene is one of the materials that is widely studied because of its unique properties. In several studies, this material has been proven theoretically and experimentally to increase the sensitivity of SPR. This paper discusses the current development of a graphene-based SPR biosensor for various excitation methods. The discussion begins with a discussion regarding the properties of graphene in general and its use in biosensors. Simulation and experimental results of several excitation methods are presented. Furthermore, the discussion regarding the SPR biosensor is expanded by providing a review regarding graphene-based Surface-Enhanced Raman Scattering (SERS) biosensor to provide an overview of the development of materials in the biosensor in the future.
Keywords: biosensors; graphene; surface plasmon resonance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













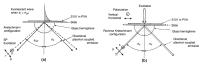




Similar articles
-
Exploring Graphene and MoS2 Chips Based Surface Plasmon Resonance Biosensors for Diagnostic Applications.Front Chem. 2020 Aug 26;8:728. doi: 10.3389/fchem.2020.00728. eCollection 2020. Front Chem. 2020. PMID: 33005604 Free PMC article. Review.
-
Optical Biosensor Based on Graphene and Its Derivatives for Detecting Biomolecules.Int J Mol Sci. 2022 Sep 16;23(18):10838. doi: 10.3390/ijms231810838. Int J Mol Sci. 2022. PMID: 36142748 Free PMC article. Review.
-
High-Sensitive Assay of Nucleic Acid Using Tetrahedral DNA Probes and DNA Concatamers with a Surface-Enhanced Raman Scattering/Surface Plasmon Resonance Dual-Mode Biosensor Based on a Silver Nanorod-Covered Silver Nanohole Array.ACS Appl Mater Interfaces. 2020 Jul 15;12(28):31242-31254. doi: 10.1021/acsami.0c08453. Epub 2020 Jul 1. ACS Appl Mater Interfaces. 2020. PMID: 32608960
-
An Au Nanofilm-Graphene/D-Type Fiber Surface Plasmon Resonance Sensor for Highly Sensitive Specificity Bioanalysis.Sensors (Basel). 2020 Feb 12;20(4):991. doi: 10.3390/s20040991. Sensors (Basel). 2020. PMID: 32059555 Free PMC article.
-
Current status of optical fiber biosensor based on surface plasmon resonance.Biosens Bioelectron. 2019 Oct 1;142:111505. doi: 10.1016/j.bios.2019.111505. Epub 2019 Jul 16. Biosens Bioelectron. 2019. PMID: 31357154 Review.
Cited by
-
Review of Gold Nanoparticles in Surface Plasmon-Coupled Emission Technology: Effect of Shape, Hollow Nanostructures, Nano-Assembly, Metal-Dielectric and Heterometallic Nanohybrids.Nanomaterials (Basel). 2024 Jan 2;14(1):111. doi: 10.3390/nano14010111. Nanomaterials (Basel). 2024. PMID: 38202566 Free PMC article. Review.
-
New Shear Horizontal (SH) Surface-Plasmon-Polariton-like Elastic Surface Waves for Sensing Applications.Sensors (Basel). 2023 Dec 17;23(24):9879. doi: 10.3390/s23249879. Sensors (Basel). 2023. PMID: 38139725 Free PMC article.
-
Carbon Dots: Classification, Properties, Synthesis, Characterization, and Applications in Health Care-An Updated Review (2018-2021).Nanomaterials (Basel). 2021 Sep 27;11(10):2525. doi: 10.3390/nano11102525. Nanomaterials (Basel). 2021. PMID: 34684966 Free PMC article. Review.
-
Intensity Interrogation-Based High-Sensitivity Surface Plasmon Resonance Imaging Biosensor for Apoptosis Detection in Cancer.Biosensors (Basel). 2023 Oct 23;13(10):946. doi: 10.3390/bios13100946. Biosensors (Basel). 2023. PMID: 37887139 Free PMC article.
-
Transformative biomedical devices to overcome biomatrix effects.Biosens Bioelectron. 2025 Jul 1;279:117373. doi: 10.1016/j.bios.2025.117373. Epub 2025 Mar 12. Biosens Bioelectron. 2025. PMID: 40120290 Review.
References
-
- Papageorgiou D.G., Kinloch I.A., Young R.J. Graphene/elastomer nanocomposites. Carbon. 2015;95:460–484. doi: 10.1016/j.carbon.2015.08.055. - DOI
-
- Bai L., Zhang Y., Tong W., Sun L., Huang H., An Q., Tian N., Chu P.K. Graphene for Energy Storage and Conversion: Synthesis and Interdisciplinary Applications. Electrochem. Energy Rev. 2020;3:395–430. doi: 10.1007/s41918-019-00042-6. - DOI
-
- Lin J., Huang Y., Huang P. Biomedical Applications of Functionalized Nanomaterials. Elsevier; Amsterdam, The Netherlands: 2018. Graphene-Based Nanomaterials in Bioimaging; pp. 247–287.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

