A phase I trial evaluating the effects of plerixafor, G-CSF, and azacitidine for the treatment of myelodysplastic syndromes
- PMID: 33467957
- PMCID: PMC8178174
- DOI: 10.1080/10428194.2021.1872068
A phase I trial evaluating the effects of plerixafor, G-CSF, and azacitidine for the treatment of myelodysplastic syndromes
Abstract
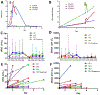
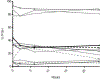
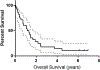
Interactions between the bone marrow microenvironment and MDS tumor clones play a role in pathogenesis and response to treatment. We hypothesized G-CSF and plerixafor may enhance sensitivity to azacitidine in MDS. Twenty-eight patients with MDS were treated with plerixafor, G-CSF and azacitidine with a standard 3 + 3 design. Subjects received G-CSF 10 mcg/kg D1-D8, plerixafor D4-D8, and azacitidine 75 mg/m2 D4-D8, but the trial was amended to reduce G-CSF dose to 5 mcg/kg for 5 days after 2 patients had significant leukocytosis. Plerixafor was dose escalated to 560 mcg/kg/day without dose limiting toxicity. Two complete responses and 6 marrow responses were seen for an overall response rate (ORR) of 36% in evaluable patients, and ORR of 53% in patients receiving the triplet. Evidence of mobilization correlated with a higher ORR, 60% vs. 17%. Plerixafor, G-CSF and azacitidine appears tolerable when given over 5 days and has encouraging response rates.KEY POINTSPlerixafor and G-CSF can be safely combined with azacitidine for 5 days in patients with MDS.The overall response rate of 53% for evaluable patients with this regimen is higher than expected and more responses were seen in patients with blast mobilization.
Keywords: G-CSF; MDS; Plerixafor; azacitidine.
Conflict of interest statement
Conflict of Interest:
EH, TF, JR, LG, KM, SC, WCE, KT, RR, SK, AG, CA, AFC, KSG, RV, PW and JFD have declared that no relevant conflict of interest exists. MAS, MPR, GLU, and RV report receiving honoraria from Sanofi. RV has received honoraria from Bristol Myers Squibb.
Figures
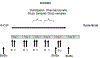
References
-
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009. March;10(3):223–32. doi: 10.1016/S1470-2045(09)70003-8. - DOI - PMC - PubMed
-
- Blau O Bone marrow stromal cells in the pathogenesis of acute myeloid leukemia. Front Biosci (Landmark Ed) 2014;19:171–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
