Abnormal eye movements in spinocerebellar ataxia type 3
- PMID: 33468086
- PMCID: PMC7814728
- DOI: 10.1186/s12883-021-02057-3
Abnormal eye movements in spinocerebellar ataxia type 3
Abstract
Background: Abnormal eye movements are common in spinocerebellar ataxias Type 3 (SCA3). We conducted the research to explore the frequency of abnormal eye movements in Chinese patients with SCA3, to compare the demographic and clinical characteristics between SCA3 patients with and without each type of abnormal eye movement, and to explore the correlation between abnormal eye movements and the severity of ataxia.
Methods: Seventy-four patients with SCA3 were enrolled in this cross-sectional study. Six types of abnormal eye movements including impaired smooth pursuit, increased square-wave jerks (SWJ), gaze-evoked nystagmus (GEN), slowing of saccades, saccadic hypo/hypermetria and supranuclear gaze palsy were evaluated by experienced neurologists. The severity of ataxia was evaluated by Scale for the Assessment and Rating of Ataxia (SARA).
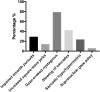
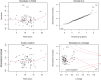
Results: The prevalence of impaired smooth pursuit, increased SWJ, GEN, slowing of saccades, saccadic hypo/hypermetria and supranuclear gaze palsy in Chinese SCA3 patients was 28.4, 13.5, 78.4, 41.9, 23.0, and 5.4%, respectively. SCA3 patients with GEN had higher scores of International Cooperative Ataxia Rating Scale (ICARS-IV) and total ICARS, and longer length of CAG repeat than patients without GEN. SCA3 patients with slowing of saccades had a longer disease duration, higher scores of ICARS-I, ICARS-II, total ICARS and SARA than patients without slowing of saccades. SCA3 patients with saccadic hypo/hypermetria had higher scores of ICARS-III, ICARS-IV, and SARA than patients without saccadic hypo/hypermetria. The demographic and clinical characteristics did not differ significantly between SCA3 patients with and without impaired smooth pursuit, increased SWJ, or supranuclear gaze palsy. Multivariate linear regression showed that the number of abnormal eye movements (0-6), disease duration, Hamilton Depression Rating Scale-24 (HDRS-24) score, and CAG repeat length were positively correlated with SARA score, whereas Montreal Cognitive Assessment (MoCA) score was negatively correlated with SARA score in SCA3.
Conclusions: An increased number of abnormal eye movement types correlated with the severity of ataxia in SCA3.
Keywords: Eye movements; Severity; Spinocerebellar Ataxia type 3.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

