The role of capecitabine-based neoadjuvant and adjuvant chemotherapy in early-stage triple-negative breast cancer: a systematic review and meta-analysis
- PMID: 33468087
- PMCID: PMC7816481
- DOI: 10.1186/s12885-021-07791-y
The role of capecitabine-based neoadjuvant and adjuvant chemotherapy in early-stage triple-negative breast cancer: a systematic review and meta-analysis
Abstract
Background: The role of capecitabine in neoadjuvant and adjuvant chemotherapy for early-stage triple-negative breast cancer (TNBC) is highly controversial. Our meta-analysis was designed to further elucidate the effects of capecitabine on survival in early-stage TNBC patients and its safety.
Methods: PubMed, Embase, and papers presented at several main conferences were searched up to December 19, 2019, to investigate capecitabine-based versus capecitabine-free neoadjuvant and adjuvant chemotherapy in TNBC patients. Heterogeneity was assessed using I2 test, combined with hazard ratios (HRs) and odds ratios (ORs) with 95% confidence intervals (CI) computed for disease-free survival (DFS), overall survival (OS), and over grade 3 adverse events (AEs).
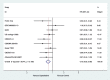
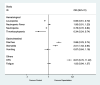
Results: A total of 9 randomized clinical trials and 3842 TNBC patients were included. Overall, the combined capecitabine regimens in neoadjuvant and adjuvant chemotherapy showed significantly improved DFS (HR = 0.75; 95% CI, 0.65-0.86; P < 0.001) and OS (HR = 0.63; 95% CI, 0.53-0.77; P < 0.001). In subgroup analysis, there were improvements in DFS in the groups with addition of capecitabine (HR = 0.64; 95% CI, 0.53-0.78; P < 0.001), adjuvant chemotherapy (HR = 0.73; 95% CI, 0.63-0.85; P < 0.001), and lymph node positivity (HR = 0.62; 95% CI, 0.44-0.86; P = 0.005). Capecitabine regimens were related to higher risks of diarrhea (OR = 2.88, 95% CI 2.23-3.74, P < 0.001), stomatitis (OR = 2.01, 95% CI 1.53-2.64, P < 0.001) and hand-foot syndrome (OR = 8.67, 95% CI 6.70-11.22, P < 0.001).
Conclusion: This meta-analysis showed that neoadjuvant and adjuvant chemotherapy combined with capecitabine significantly improved both DFS and OS in early-stage TNBC patients with tolerable AEs. There were benefits to DFS in the groups with the addition of capecitabine, adjuvant chemotherapy, and lymph node positivity.
Keywords: Capecitabine; Neo/adjuvant chemotherapy; Survival; Triple-negative breast cancer.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures




References
-
- Fasching PA, Jackisch C, Rhiem K, Schneeweiss A, Klare P, Hanusch C, et al. A randomized phase II trial to assess the efficacy of paclitaxel and olaparib in comparison to paclitaxel/carboplatin followed by epirubicin/cyclophosphamide as neoadjuvant chemotherapy in patients (pts) with HER2-negative early breast cancer (BC) and homologous recombination deficiency (HRD). J Clin Oncol. 2019:506.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

