Activation of MC1R with BMS-470539 attenuates neuroinflammation via cAMP/PKA/Nurr1 pathway after neonatal hypoxic-ischemic brain injury in rats
- PMID: 33468172
- PMCID: PMC7814630
- DOI: 10.1186/s12974-021-02078-2
Activation of MC1R with BMS-470539 attenuates neuroinflammation via cAMP/PKA/Nurr1 pathway after neonatal hypoxic-ischemic brain injury in rats
Abstract
Background: Microglia-mediated neuroinflammation plays a crucial role in the pathogenesis of hypoxic-ischemic (HI)-induced brain injury. Activation of melanocortin-1 receptor (MC1R) has been shown to exert anti-inflammatory and neuroprotective effects in several neurological diseases. In the present study, we have explored the role of MC1R activation on neuroinflammation and the potential underlying mechanisms after neonatal hypoxic-ischemic brain injury in rats.
Methods: A total of 169 post-natal day 10 unsexed rat pups were used. HI was induced by right common carotid artery ligation followed by 2.5 h of hypoxia. BMS-470539, a specific selective MC1R agonist, was administered intranasally at 1 h after HI induction. To elucidate the potential underlying mechanism, MC1R CRISPR KO plasmid or Nurr1 CRISPR KO plasmid was administered via intracerebroventricular injection at 48 h before HI induction. Percent brain infarct area, short- and long-term neurobehavioral tests, Nissl staining, immunofluorescence staining, and Western blot were conducted.
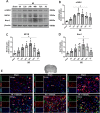
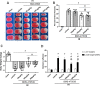
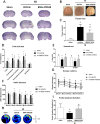
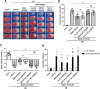
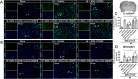
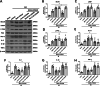
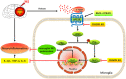
Results: The expression levels of MC1R and Nurr1 increased over time post-HI. MC1R and Nurr1 were expressed on microglia at 48 h post-HI. Activation of MC1R with BMS-470539 significantly reduced the percent infarct area, brain atrophy, and inflammation, and improved short- and long-term neurological deficits at 48 h and 28 days post-HI. MC1R activation increased the expression of CD206 (a microglial M2 marker) and reduced the expression of MPO. Moreover, activation of MC1R with BMS-470539 significantly increased the expression levels of MC1R, cAMP, p-PKA, and Nurr1, while downregulating the expression of pro-inflammatory cytokines (TNFα, IL-6, and IL-1β) at 48 h post-HI. However, knockout of MC1R or Nurr1 by specific CRISPR reversed the neuroprotective effects of MC1R activation post-HI.
Conclusions: Our study demonstrated that activation of MC1R with BMS-470539 attenuated neuroinflammation, and improved neurological deficits after neonatal hypoxic-ischemic brain injury in rats. Such anti-inflammatory and neuroprotective effects were mediated, at least in part, via the cAMP/PKA/Nurr1 signaling pathway. Therefore, MC1R activation might be a promising therapeutic target for infants with hypoxic-ischemic encephalopathy (HIE).
Keywords: BMS-470539; Melanocortin-1 receptor; Microglial polarization; Neonatal hypoxia-ischemia; Neuroinflammation; Nurr1.
Conflict of interest statement
All the authors declared no conflicts of interest.
Figures


Similar articles
-
BMS-470539 Attenuates Oxidative Stress and Neuronal Apoptosis via MC1R/cAMP/PKA/Nurr1 Signaling Pathway in a Neonatal Hypoxic-Ischemic Rat Model.Oxid Med Cell Longev. 2022 Jan 31;2022:4054938. doi: 10.1155/2022/4054938. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35140838 Free PMC article.
-
Rh-CSF1 attenuates neuroinflammation via the CSF1R/PLCG2/PKCε pathway in a rat model of neonatal HIE.J Neuroinflammation. 2020 Jun 10;17(1):182. doi: 10.1186/s12974-020-01862-w. J Neuroinflammation. 2020. PMID: 32522286 Free PMC article.
-
Activation of GPR39 with TC-G 1008 attenuates neuroinflammation via SIRT1/PGC-1α/Nrf2 pathway post-neonatal hypoxic-ischemic injury in rats.J Neuroinflammation. 2021 Oct 13;18(1):226. doi: 10.1186/s12974-021-02289-7. J Neuroinflammation. 2021. PMID: 34645465 Free PMC article.
-
Hydrogen-rich saline promotes microglia M2 polarization and complement-mediated synapse loss to restore behavioral deficits following hypoxia-ischemic in neonatal mice via AMPK activation.J Neuroinflammation. 2019 May 18;16(1):104. doi: 10.1186/s12974-019-1488-2. J Neuroinflammation. 2019. PMID: 31103039 Free PMC article. Review.
-
The role of G-CSF neuroprotective effects in neonatal hypoxic-ischemic encephalopathy (HIE): current status.J Neuroinflammation. 2021 Feb 21;18(1):55. doi: 10.1186/s12974-021-02084-4. J Neuroinflammation. 2021. PMID: 33612099 Free PMC article. Review.
Cited by
-
Melanocortin therapies to resolve fibroblast-mediated diseases.Front Immunol. 2023 Jan 30;13:1084394. doi: 10.3389/fimmu.2022.1084394. eCollection 2022. Front Immunol. 2023. PMID: 36793548 Free PMC article. Review.
-
The Melanocortin System: A Promising Target for the Development of New Antidepressant Drugs.Int J Mol Sci. 2023 Apr 3;24(7):6664. doi: 10.3390/ijms24076664. Int J Mol Sci. 2023. PMID: 37047638 Free PMC article. Review.
-
Mitochondrial-targeted therapies in traumatic brain injury: From bench to bedside.Neurotherapeutics. 2025 Jan;22(1):e00515. doi: 10.1016/j.neurot.2024.e00515. Epub 2024 Dec 24. Neurotherapeutics. 2025. PMID: 39721917 Free PMC article. Review.
-
Modulation of Second Messenger Signaling in the Brain Through PDE4 and PDE5 Inhibition: Therapeutic Implications for Neurological Disorders.Cells. 2025 Jan 9;14(2):86. doi: 10.3390/cells14020086. Cells. 2025. PMID: 39851514 Free PMC article. Review.
-
Berberine mitigates colitis-associated neuroinflammation and anxiety through modulation of the AMPK/NURR1 pathway.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun 26. doi: 10.1007/s00210-025-04376-5. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40569370 Review.
References
-
- Shaywitz BA, Fletcher JM. Neurological, cognitive, and behavioral sequelae of hypoxic-ischemic encephalopathy. Semin Perinatol. 1993;17(5):357–366. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

