Red and fallow deer determine the density of Ixodes ricinus nymphs containing Anaplasma phagocytophilum
- PMID: 33468215
- PMCID: PMC7814456
- DOI: 10.1186/s13071-020-04567-4
Red and fallow deer determine the density of Ixodes ricinus nymphs containing Anaplasma phagocytophilum
Abstract
Background: The density of Ixodes ricinus nymphs infected with Anaplasma phagocytophilum is one of the parameters that determines the risk for humans and domesticated animals to contract anaplasmosis. For this, I. ricinus larvae need to take a bloodmeal from free-ranging ungulates, which are competent hosts for A. phagocytophilum.
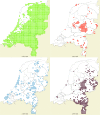
Methods: Here, we compared the contribution of four free-ranging ungulate species, red deer (Cervus elaphus), fallow deer (Dama dama), roe deer (Capreolus capreolus), and wild boar (Sus scrofa), to A. phagocytophilum infections in nymphs. We used a combination of camera and live trapping to quantify the relative availability of vertebrate hosts to questing ticks in 19 Dutch forest sites. Additionally, we collected questing I. ricinus nymphs and tested these for the presence of A. phagocytophilum. Furthermore, we explored two potential mechanisms that could explain differences between species: (i) differences in larval burden, which we based on data from published studies, and (ii) differences in associations with other, non-competent hosts.
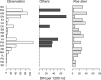
Results: Principal component analysis indicated that the density of A. phagocytophilum-infected nymphs (DIN) was higher in forest sites with high availability of red and fallow deer, and to a lesser degree roe deer. Initial results suggest that these differences are not a result of differences in larval burden, but rather differences in associations with other species or other ecological factors.
Conclusions: These results indicate that the risk for contracting anaplasmosis in The Netherlands is likely highest in the few areas where red and fallow deer are present. Future studies are needed to explore the mechanisms behind this association.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Wild ungulate species differ in their contribution to the transmission of Ixodes ricinus-borne pathogens.Parasit Vectors. 2021 Jul 10;14(1):360. doi: 10.1186/s13071-021-04860-w. Parasit Vectors. 2021. PMID: 34246293 Free PMC article.
-
Diverse tick-borne microorganisms identified in free-living ungulates in Slovakia.Parasit Vectors. 2018 Sep 3;11(1):495. doi: 10.1186/s13071-018-3068-1. Parasit Vectors. 2018. PMID: 30176908 Free PMC article.
-
The role of different species of wild ungulates and Ixodes ricinus ticks in the circulation of genetic variants of Anaplasma phagocytophilum in a forest biotope in north-western Poland.Ticks Tick Borne Dis. 2020 Sep;11(5):101465. doi: 10.1016/j.ttbdis.2020.101465. Epub 2020 May 16. Ticks Tick Borne Dis. 2020. PMID: 32723651
-
Infection prevalence and ecotypes of Anaplasma phagocytophilum in moose Alces alces, red deer Cervus elaphus, roe deer Capreolus capreolus and Ixodes ricinus ticks from Norway.Parasit Vectors. 2019 Jan 3;12(1):1. doi: 10.1186/s13071-018-3256-z. Parasit Vectors. 2019. PMID: 30606222 Free PMC article.
-
[Ixodes ricinus, transmitted diseases and reservoirs].Parassitologia. 2004 Jun;46(1-2):119-22. Parassitologia. 2004. PMID: 15305699 Review. Italian.
Cited by
-
Bacteria and protozoa with pathogenic potential in Ixodes ricinus ticks in Viennese recreational areas.Wien Klin Wochenschr. 2023 Apr;135(7-8):177-184. doi: 10.1007/s00508-022-02046-7. Epub 2022 Jun 10. Wien Klin Wochenschr. 2023. PMID: 35689113 Free PMC article.
-
Tick Activity, Host Range, and Tick-Borne Pathogen Prevalence in Mountain Habitats of the Western Carpathians, Poland.Pathogens. 2023 Sep 21;12(9):1186. doi: 10.3390/pathogens12091186. Pathogens. 2023. PMID: 37764994 Free PMC article.
-
Assessing zoonotic risk in a fenced natural park in northwestern Italy: integrating camera traps for a vector-host approach to investigate tick-borne pathogens.Front Vet Sci. 2025 Mar 3;12:1536260. doi: 10.3389/fvets.2025.1536260. eCollection 2025. Front Vet Sci. 2025. PMID: 40098889 Free PMC article.
-
Is composition of vertebrates an indicator of the prevalence of tick-borne pathogens?Infect Ecol Epidemiol. 2022 Jan 10;12(1):2025647. doi: 10.1080/20008686.2022.2025647. eCollection 2022. Infect Ecol Epidemiol. 2022. PMID: 35035783 Free PMC article.
-
Novel Protozoans in Austria Revealed through the Use of Dogs as Sentinels for Ticks and Tick-Borne Pathogens.Microorganisms. 2021 Jun 28;9(7):1392. doi: 10.3390/microorganisms9071392. Microorganisms. 2021. PMID: 34203236 Free PMC article.
References
-
- Hovius E, de Bruin A, Schouls L, Hovius J, Dekker N, Sprong H. A lifelong study of a pack Rhodesian ridgeback dogs reveals subclinical and clinical tick-borne Anaplasma phagocytophilum infections with possible reinfection or persistence. Parasit Vectors. 2018;11:238. doi: 10.1186/s13071-018-2806-8. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

