Analysis of health claims regarding creatine monohydrate present in commercial communications for a sample of European sports foods supplements
- PMID: 33468268
- PMCID: PMC11574825
- DOI: 10.1017/S1368980020005121
Analysis of health claims regarding creatine monohydrate present in commercial communications for a sample of European sports foods supplements
Abstract
Objective: To analyse the information on health claims present in the labelling of creatine monohydrate (CM) products.
Design: A descriptive study of a selection of products marketed as CM, with health claims, and that met the inclusion/exclusion criteria, was conducted using the Amazon and Google Shopping websites. The adequacy and compliance of the health claims were evaluated with the European legislative requirements (European Food Safety Authority and European Commission). The results were discussed with scientific evidence criteria from the Academy of Nutrition and Dietetics, International Olympic Committee, and International Society of Sports Nutrition, as well as the systematic review carried out by Mielgo-Ayuso in 2019.
Setting: Health claims in the commercial communications of a sample of CM supplements, in relation to current legislation and scientific knowledge.
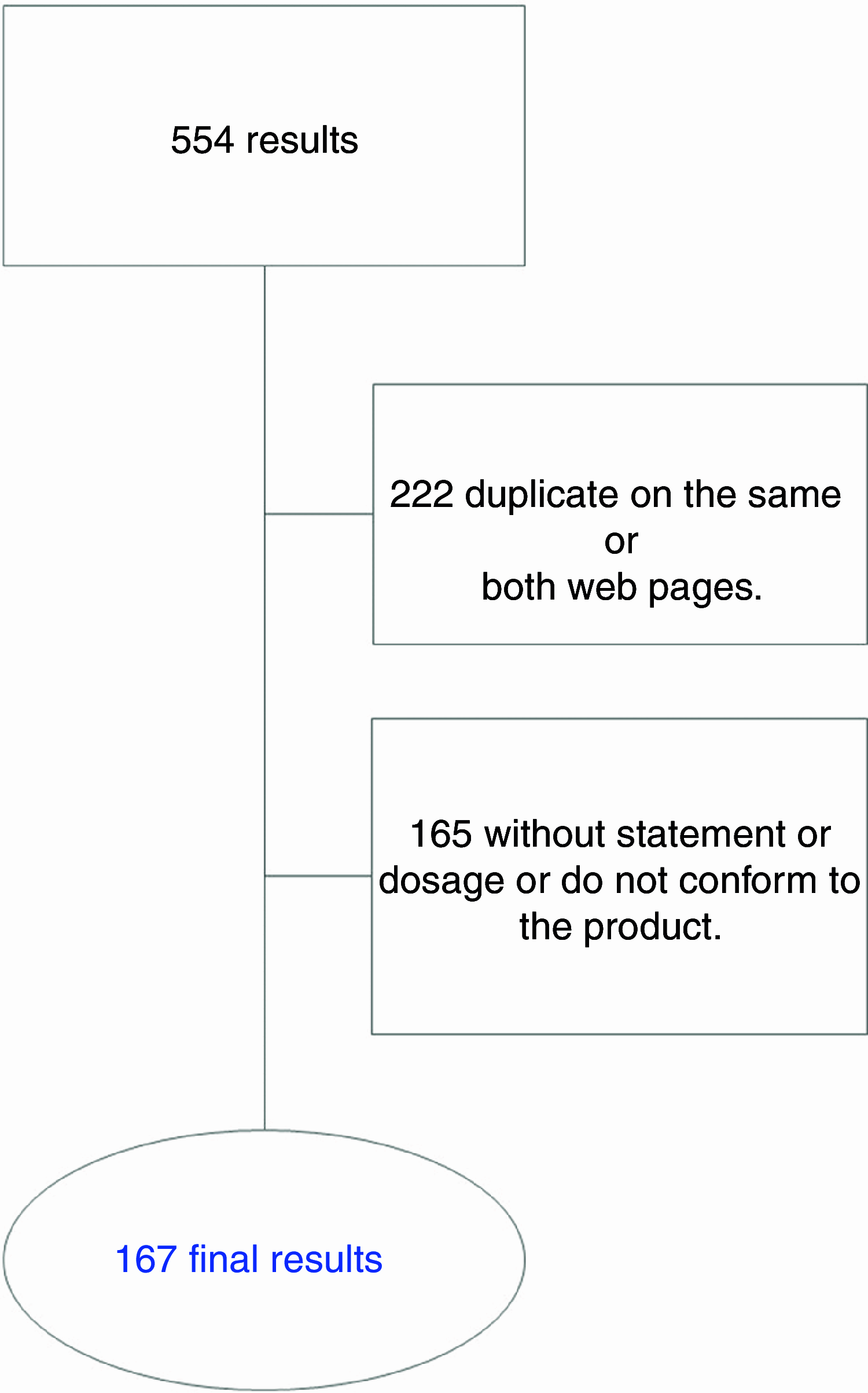
Participant: A total of 554 CM products were obtained.
Results: Of the total sample, only 167 met the inclusion/exclusion criteria. Of these, 30·5 % recommended a CM dose of 5·0-5·9 g/d, while 29·9 % recommended 3·0 to 3·9 g/d. As for the health claims, 'Enhances physical performance' appeared in 73·1 % of the supplements, in most cases referring to a dosage of 3·0 to 3·9 g/d for these products. The rest of the declarations were not adequate or needed to be modified.
Conclusion: Only 25 % of the health claims complied with the criteria established by the scientific reference documents. Most of the declarations must be modified or eliminated, as they could be considered fraudulent and/or misleading for the consumer.
Keywords: Creatine; Health claims; Nutrition; Nutritional labelling; Sports performance.
Figures
Similar articles
-
Health Claims for Sports Drinks-Analytical Assessmentaccording to European Food Safety Authority's Scientific Opinion.Nutrients. 2024 Jun 21;16(13):1980. doi: 10.3390/nu16131980. Nutrients. 2024. PMID: 38999728 Free PMC article.
-
Caffeine Health Claims on Sports Supplement Labeling. Analytical Assessment According to EFSA Scientific Opinion and International Evidence and Criteria.Molecules. 2021 Apr 6;26(7):2095. doi: 10.3390/molecules26072095. Molecules. 2021. PMID: 33917477 Free PMC article.
-
Current Status of Legislation on Dietary Products for Sportspeople in a European Framework.Nutrients. 2017 Nov 8;9(11):1225. doi: 10.3390/nu9111225. Nutrients. 2017. PMID: 29117104 Free PMC article. Review.
-
European regulations on nutraceuticals, dietary supplements and functional foods: a framework based on safety.Toxicology. 2006 Apr 3;221(1):59-74. doi: 10.1016/j.tox.2005.12.022. Epub 2006 Feb 15. Toxicology. 2006. PMID: 16469424
-
Creatine as nutritional supplementation and medicinal product.J Sports Med Phys Fitness. 2001 Mar;41(1):1-10. J Sports Med Phys Fitness. 2001. PMID: 11317142 Review.
Cited by
-
Health Claims for Protein Food Supplements for Athletes-The Analysis Is in Accordance with the EFSA's Scientific Opinion.Nutrients. 2025 Jun 3;17(11):1923. doi: 10.3390/nu17111923. Nutrients. 2025. PMID: 40507192 Free PMC article.
-
Sports foods are not all they shake up to be. An audit of formulated supplementary sports food products and packaging in Australian retail environments.Front Nutr. 2023 Feb 14;10:1042049. doi: 10.3389/fnut.2023.1042049. eCollection 2023. Front Nutr. 2023. PMID: 36866048 Free PMC article.
-
The Prevalence and Compliance of Health Claims Used in the Labelling and Information for Prepacked Foods within Great Britain.Foods. 2024 Feb 9;13(4):539. doi: 10.3390/foods13040539. Foods. 2024. PMID: 38397515 Free PMC article.
-
A Cross-Sectional Study of Sports Food Consumption Patterns, Experiences, and Perceptions amongst Non-Athletes in Australia.Nutrients. 2024 Apr 9;16(8):1101. doi: 10.3390/nu16081101. Nutrients. 2024. PMID: 38674792 Free PMC article.
-
Health Claims for Sports Drinks-Analytical Assessmentaccording to European Food Safety Authority's Scientific Opinion.Nutrients. 2024 Jun 21;16(13):1980. doi: 10.3390/nu16131980. Nutrients. 2024. PMID: 38999728 Free PMC article.
References
-
- Palacios Gil de Antuñano N & Manonelles Marqueta P (2012) Ergogenic nutritional aids for people who perform physical exercise: consensus document of the Spanish Federation of Sports Medicine. Archivos Med Deporte 39, 76.
-
- Moriones VS & Santos JI (2017) Ergogenic aids in sport. Nutricion Hospitalaria 34, 204–215. - PubMed
-
- Maughan RJ, Greenhaff PL & Hespel P (2011) Dietary supplements for athletes: emerging trends and recurring themes. J Sports Sci 29, S57–S66. - PubMed
-
- Garthe I & Maughan RJ (2018) Athletes and supplements: prevalence and perspectives. Int J Sport Nutr Exercise Metab 28, 126–138. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous