Boromycin Has Potent Anti- Toxoplasma and Anti- Cryptosporidium Activity
- PMID: 33468470
- PMCID: PMC8097477
- DOI: 10.1128/AAC.01278-20
Boromycin Has Potent Anti- Toxoplasma and Anti- Cryptosporidium Activity
Abstract
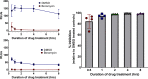
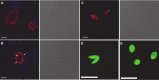
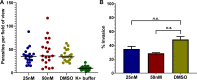
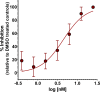
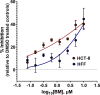
Toxoplasma gondii and Cryptosporidium parvum, members of the phylum Apicomplexa, are significant pathogens of both humans and animals worldwide for which new and effective therapeutics are needed. Here, we describe the activity of the antibiotic boromycin against Toxoplasma and Cryptosporidium Boromycin potently inhibited intracellular proliferation of both T. gondii and C. parvum at half-maximal effective concentrations (EC50) of 2.27 nM and 4.99 nM, respectively. Treatment of extracellular T. gondii tachyzoites with 25 nM boromycin for 30 min suppressed 84% of parasite growth, but T. gondii tachyzoite invasion into host cells was not affected by boromycin. Immunofluorescence of boromycin-treated T. gondii showed loss of morphologically intact parasites with randomly distributed surface antigens inside the parasitophorous vacuoles. Boromycin exhibited a high selectivity for the parasites over their host cells. These results suggest that boromycin is a promising new drug candidate for treating toxoplasmosis and cryptosporidiosis.
Keywords: Cryptosporidium parvum; Toxoplasma gondii; antiparasitic; boromycin; drug discovery.
Copyright © 2021 American Society for Microbiology.
Figures

Similar articles
-
Activity of (1-benzyl-4-triazolyl)-indole-2-carboxamides against Toxoplasma gondii and Cryptosporidium parvum.Int J Parasitol Drugs Drug Resist. 2022 Aug;19:6-20. doi: 10.1016/j.ijpddr.2022.04.001. Epub 2022 Apr 16. Int J Parasitol Drugs Drug Resist. 2022. PMID: 35462232 Free PMC article.
-
Novel acyl carbamates and acyl / diacyl ureas show in vitro efficacy against Toxoplasma gondii and Cryptosporidium parvum.Int J Parasitol Drugs Drug Resist. 2020 Dec;14:80-90. doi: 10.1016/j.ijpddr.2020.08.006. Epub 2020 Aug 25. Int J Parasitol Drugs Drug Resist. 2020. PMID: 33011650 Free PMC article.
-
In Vitro and In Vivo Activities of Tilmicosin and Acetylisovaleryltylosin Tartrate against Toxoplasma gondii.Int J Mol Sci. 2022 Aug 24;23(17):9586. doi: 10.3390/ijms23179586. Int J Mol Sci. 2022. PMID: 36076987 Free PMC article.
-
Apicomplexan infections in the gut.Parasite Immunol. 2014 Sep;36(9):409-20. doi: 10.1111/pim.12115. Parasite Immunol. 2014. PMID: 25201405 Review.
-
In vitro cultivation methods for coccidian parasite research.Int J Parasitol. 2023 Aug;53(9):477-489. doi: 10.1016/j.ijpara.2022.10.002. Epub 2022 Nov 15. Int J Parasitol. 2023. PMID: 36400306 Review.
Cited by
-
Construction of luciferase-expressing Neospora caninum and drug screening.Parasit Vectors. 2024 Mar 8;17(1):118. doi: 10.1186/s13071-024-06195-8. Parasit Vectors. 2024. PMID: 38459572 Free PMC article.
-
Diagnosis and control of cryptosporidiosis in farm animals.J Parasit Dis. 2022 Dec;46(4):1133-1146. doi: 10.1007/s12639-022-01513-2. Epub 2022 Jul 4. J Parasit Dis. 2022. PMID: 36457776 Free PMC article. Review.
-
Boromycin has Rapid-Onset Antibiotic Activity Against Asexual and Sexual Blood Stages of Plasmodium falciparum.Front Cell Infect Microbiol. 2022 Jan 14;11:802294. doi: 10.3389/fcimb.2021.802294. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35096650 Free PMC article.
-
Tartrolon E rapidly blocks Toxoplasma gondii capacity to invade host cells.Int J Parasitol Drugs Drug Resist. 2025 Aug;28:100591. doi: 10.1016/j.ijpddr.2025.100591. Epub 2025 Apr 14. Int J Parasitol Drugs Drug Resist. 2025. PMID: 40233443 Free PMC article.
-
Activity of Organoboron Compounds against Biofilm-Forming Pathogens.Antibiotics (Basel). 2024 Sep 29;13(10):929. doi: 10.3390/antibiotics13100929. Antibiotics (Basel). 2024. PMID: 39452196 Free PMC article. Review.
References
-
- Piekarski G. 1981. Behavioral alterations caused by parasitic infection in case of latent toxoplasma infection. Zentralbl Bakteriol Mikrobiol Hyg A 250:403–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

