Identification of Antifungal Compounds against Multidrug-Resistant Candida auris Utilizing a High-Throughput Drug-Repurposing Screen
- PMID: 33468482
- PMCID: PMC8097445
- DOI: 10.1128/AAC.01305-20
Identification of Antifungal Compounds against Multidrug-Resistant Candida auris Utilizing a High-Throughput Drug-Repurposing Screen
Abstract
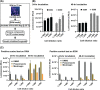
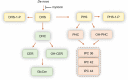
Candida auris is an emerging fatal fungal infection that has resulted in several outbreaks in hospitals and care facilities. Current treatment options are limited by the development of drug resistance. Identification of new pharmaceuticals to combat these drug-resistant infections will thus be required to overcome this unmet medical need. We have established a bioluminescent ATP-based assay to identify new compounds and potential drug combinations showing effective growth inhibition against multiple strains of multidrug-resistant Candida auris The assay is robust and suitable for assessing large compound collections by high-throughput screening (HTS). Utilizing this assay, we conducted a screen of 4,314 approved drugs and pharmacologically active compounds that yielded 25 compounds, including 6 novel anti-Candida auris compounds and 13 sets of potential two-drug combinations. Among the drug combinations, the serine palmitoyltransferase inhibitor myriocin demonstrated a combinational effect with flucytosine against all tested isolates during screening. This combinational effect was confirmed in 13 clinical isolates of Candida auris.
Keywords: Candida auris; drug combination therapy; drug repurposing; multidrug resistance.
Figures

References
-
- Magill SS, O’Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, Wilson LE, Kainer MA, Lynfield R, Greissman S, Ray SM, Beldavs Z, Gross C, Bamberg W, Sievers M, Concannon C, Buhr N, Warnke L, Maloney M, Ocampo V, Brooks J, Oyewumi T, Sharmin S, Richards K, Rainbow J, Samper M, Hancock EB, Leaptrot D, Scalise E, Badrun F, Phelps R, Edwards JR. 2018. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med 379:1732–1744. doi: 10.1056/NEJMoa1801550. - DOI - PMC - PubMed
-
- Tsay S, Williams S, Mu Y, Epson E, Johnston H, Farley MM, Harrison LH, Vonbank B, Shrum S, Dumyati G, Zhang A, Schaffner W, Magill S, Vallabhaneni S. 2018. National burden of candidemia, United States, 2017. Open Forum Infect Dis 5:S142–3. doi: 10.1093/ofid/ofy210.374. - DOI
-
- Morgan J, Meltzer MI, Plikaytis BD, Sofair AN, Huie-White S, Wilcox S, Harrison LH, Seaberg EC, Hajjeh RA, Teutsch SM. 2005. Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect Control Hosp Epidemiol 26:540–547. doi: 10.1086/502581. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

