Home and Online Management and Evaluation of Blood Pressure (HOME BP) using a digital intervention in poorly controlled hypertension: randomised controlled trial
- PMID: 33468518
- PMCID: PMC7814507
- DOI: 10.1136/bmj.m4858
Home and Online Management and Evaluation of Blood Pressure (HOME BP) using a digital intervention in poorly controlled hypertension: randomised controlled trial
Abstract
Objective: The HOME BP (Home and Online Management and Evaluation of Blood Pressure) trial aimed to test a digital intervention for hypertension management in primary care by combining self-monitoring of blood pressure with guided self-management.
Design: Unmasked randomised controlled trial with automated ascertainment of primary endpoint.
Setting: 76 general practices in the United Kingdom.
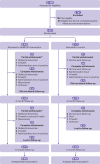
Participants: 622 people with treated but poorly controlled hypertension (>140/90 mm Hg) and access to the internet.
Interventions: Participants were randomised by using a minimisation algorithm to self-monitoring of blood pressure with a digital intervention (305 participants) or usual care (routine hypertension care, with appointments and drug changes made at the discretion of the general practitioner; 317 participants). The digital intervention provided feedback of blood pressure results to patients and professionals with optional lifestyle advice and motivational support. Target blood pressure for hypertension, diabetes, and people aged 80 or older followed UK national guidelines.
Main outcome measures: The primary outcome was the difference in systolic blood pressure (mean of second and third readings) after one year, adjusted for baseline blood pressure, blood pressure target, age, and practice, with multiple imputation for missing values.
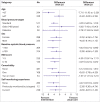
Results: After one year, data were available from 552 participants (88.6%) with imputation for the remaining 70 participants (11.4%). Mean blood pressure dropped from 151.7/86.4 to 138.4/80.2 mm Hg in the intervention group and from 151.6/85.3 to 141.8/79.8 mm Hg in the usual care group, giving a mean difference in systolic blood pressure of -3.4 mm Hg (95% confidence interval -6.1 to -0.8 mm Hg) and a mean difference in diastolic blood pressure of -0.5 mm Hg (-1.9 to 0.9 mm Hg). Results were comparable in the complete case analysis and adverse effects were similar between groups. Within trial costs showed an incremental cost effectiveness ratio of £11 ($15, €12; 95% confidence interval £6 to £29) per mm Hg reduction.
Conclusions: The HOME BP digital intervention for the management of hypertension by using self-monitored blood pressure led to better control of systolic blood pressure after one year than usual care, with low incremental costs. Implementation in primary care will require integration into clinical workflows and consideration of people who are digitally excluded.
Trial registration: ISRCTN13790648.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from NIHR for the submitted work; Omron provided the monitors used in the HOME BP study at reduced cost; RJM received BP monitors for research from Omron and is collaborating with them on development of a telemonitoring system as part of the NIHR Oxford and Thames Valley Applied Research Consortium; RJM receives occasional travel and accommodation reimbursement for talks; he does not personally receive any honorariums or consultancy payments; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224-60. 10.1016/S0140-6736(12)61766-8. - DOI - PMC - PubMed
-
- Health Survey for England 2017 Trend Tables. London: Health and Social Care Information Centre Information Centre; 2018 https://digital.nhs.uk/data-and-information/publications/statistical/hea....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
