Alkaline pH Increases Swimming Speed and Facilitates Mucus Penetration for Vibrio cholerae
- PMID: 33468594
- PMCID: PMC8088521
- DOI: 10.1128/JB.00607-20
Alkaline pH Increases Swimming Speed and Facilitates Mucus Penetration for Vibrio cholerae
Abstract
Intestinal mucus is the first line of defense against intestinal pathogens. It acts as a physical barrier between epithelial tissues and the lumen that enteropathogens must overcome to establish a successful infection. We investigated the motile behavior of two Vibrio cholerae strains (El Tor C6706 and Classical O395) in mucus using single-cell tracking in unprocessed porcine intestinal mucus. We determined that V. cholerae can penetrate mucus using flagellar motility and that alkaline pH increases swimming speed and, consequently, improves mucus penetration. Microrheological measurements indicate that changes in pH between 6 and 8 (the physiological range for the human small intestine) had little effect on the viscoelastic properties of mucus. Finally, we determined that acidic pH promotes surface attachment by activating the mannose-sensitive hemagglutinin (MshA) pilus in V. cholerae El Tor C6706 without a measurable change in the total cellular concentration of the secondary messenger cyclic dimeric GMP (c-di-GMP). Overall, our results support the hypothesis that pH is an important factor affecting the motile behavior of V. cholerae and its ability to penetrate mucus. Therefore, changes in pH along the human small intestine may play a role in determining the preferred site for V. cholerae during infection.IMPORTANCE The diarrheal disease cholera is still a burden for populations in developing countries with poor sanitation. To develop effective vaccines and prevention strategies against Vibrio cholerae, we must understand the initial steps of infection leading to the colonization of the small intestine. To infect the host and deliver the cholera toxin, V. cholerae has to penetrate the mucus layer protecting the intestinal tissues. However, the interaction of V. cholerae with intestinal mucus has not been extensively investigated. In this report, we demonstrated using single-cell tracking that V. cholerae can penetrate intestinal mucus using flagellar motility. In addition, we observed that alkaline pH improves the ability of V. cholerae to penetrate mucus. This finding has important implications for understanding the dynamics of infection, because pH varies significantly along the small intestine, between individuals, and between species. Blocking mucus penetration by interfering with flagellar motility in V. cholerae, reinforcing the mucosa, controlling intestinal pH, or manipulating the intestinal microbiome will offer new strategies to fight cholera.
Keywords: Vibrio cholerae; cell tracking; flagellar motility; intestinal mucus; microrheology; pH.
Copyright © 2021 Nhu et al.
Figures




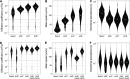
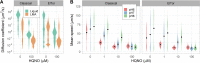
Similar articles
-
Expression of Vibrio cholerae virulence genes in response to environmental signals.Curr Issues Intest Microbiol. 2002 Sep;3(2):29-38. Curr Issues Intest Microbiol. 2002. PMID: 12400636 Review.
-
Transcriptional Silencing by TsrA in the Evolution of Pathogenic Vibrio cholerae Biotypes.mBio. 2020 Nov 24;11(6):e02901-20. doi: 10.1128/mBio.02901-20. mBio. 2020. PMID: 33234688 Free PMC article.
-
Intestinal Colonization Dynamics of Vibrio cholerae.PLoS Pathog. 2015 May 21;11(5):e1004787. doi: 10.1371/journal.ppat.1004787. eCollection 2015 May. PLoS Pathog. 2015. PMID: 25996593 Free PMC article. Review.
-
Aerobic Metabolism in Vibrio cholerae Is Required for Population Expansion during Infection.mBio. 2020 Sep 1;11(5):e01989-20. doi: 10.1128/mBio.01989-20. mBio. 2020. PMID: 32873763 Free PMC article.
-
Differential modulation of NF-kappaB-mediated pro-inflammatory response in human intestinal epithelial cells by cheY homologues of Vibrio cholerae.Innate Immun. 2009 Jun;15(3):131-42. doi: 10.1177/1753425908100454. Innate Immun. 2009. PMID: 19474207
Cited by
-
Vibrio cholerae Alkalizes Its Environment via Citrate Metabolism to Inhibit Enteric Growth In Vitro.Microbiol Spectr. 2023 Mar 14;11(2):e0491722. doi: 10.1128/spectrum.04917-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36916917 Free PMC article.
-
Vibrio cholerae Motility in Aquatic and Mucus-Mimicking Environments.Appl Environ Microbiol. 2021 Sep 28;87(20):e0129321. doi: 10.1128/AEM.01293-21. Epub 2021 Aug 4. Appl Environ Microbiol. 2021. PMID: 34347522 Free PMC article.
-
Non-O1/Non-O139 Vibrio cholerae-An Underestimated Foodborne Pathogen? An Overview of Its Virulence Genes and Regulatory Systems Involved in Pathogenesis.Microorganisms. 2024 Apr 18;12(4):818. doi: 10.3390/microorganisms12040818. Microorganisms. 2024. PMID: 38674762 Free PMC article. Review.
-
Mucus interaction to improve gastrointestinal retention and pharmacokinetics of orally administered nano-drug delivery systems.J Nanobiotechnology. 2022 Aug 6;20(1):362. doi: 10.1186/s12951-022-01539-x. J Nanobiotechnology. 2022. PMID: 35933341 Free PMC article. Review.
-
L-Ascorbic Acid Restricts Vibrio cholerae Survival in Various Growth Conditions.Microorganisms. 2024 Feb 29;12(3):492. doi: 10.3390/microorganisms12030492. Microorganisms. 2024. PMID: 38543543 Free PMC article.
References
-
- Bi Q, Ferreras E, Pezzoli L, Legros D, Ivers LC, Date K, Qadri F, Digilio L, Sack DA, Ali M, Lessler J, Luquero FJ, Azman AS, Cavailler P, Date K, Sreenivasan N, Matzger H, Luquero F, Grais R, Wiesner L, Ko M, Rouzier V, Peak C, Qadri F, Landegger J, Lynch J, Azman A, Sack D, Henkens M, Ciglenecki I, Ivers L, Diggle E, Weiss M, Hinman A, Maina K, Mirza I, Gimeno G, Levine M. 2017. Protection against cholera from killed whole-cell oral cholera vaccines: a systematic review and meta-analysis. Lancet Infect Dis 17:1080–1088. doi:10.1016/S1473-3099(17)30359-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

