Discrete TrkB-expressing neurons of the dorsomedial hypothalamus regulate feeding and thermogenesis
- PMID: 33468645
- PMCID: PMC7848633
- DOI: 10.1073/pnas.2017218118
Discrete TrkB-expressing neurons of the dorsomedial hypothalamus regulate feeding and thermogenesis
Abstract
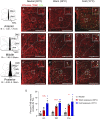
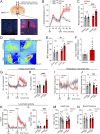
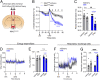
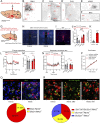
Mutations in the TrkB neurotrophin receptor lead to profound obesity in humans, and expression of TrkB in the dorsomedial hypothalamus (DMH) is critical for maintaining energy homeostasis. However, the functional implications of TrkB-fexpressing neurons in the DMH (DMHTrkB) on energy expenditure are unclear. Additionally, the neurocircuitry underlying the effect of DMHTrkB neurons on energy homeostasis has not been explored. In this study, we show that activation of DMHTrkB neurons leads to a robust increase in adaptive thermogenesis and energy expenditure without altering heart rate or blood pressure, while silencing DMHTrkB neurons impairs thermogenesis. Furthermore, we reveal neuroanatomically and functionally distinct populations of DMHTrkB neurons that regulate food intake or thermogenesis. Activation of DMHTrkB neurons projecting to the raphe pallidus (RPa) stimulates thermogenesis and increased energy expenditure, whereas DMHTrkB neurons that send collaterals to the paraventricular hypothalamus (PVH) and preoptic area (POA) inhibit feeding. Together, our findings provide evidence that DMHTrkB neuronal activity plays an important role in regulating energy expenditure and delineate distinct neurocircuits that underly the separate effects of DMHTrkB neuronal activity on food intake and thermogenesis.
Keywords: TrkB; dorsomedial hypothalamus; energy expenditure; feeding; neurocircuitry.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
TrkB-expressing neurons in the dorsomedial hypothalamus are necessary and sufficient to suppress homeostatic feeding.Proc Natl Acad Sci U S A. 2019 Feb 19;116(8):3256-3261. doi: 10.1073/pnas.1815744116. Epub 2019 Feb 4. Proc Natl Acad Sci U S A. 2019. PMID: 30718415 Free PMC article.
-
TrkB-expressing paraventricular hypothalamic neurons suppress appetite through multiple neurocircuits.Nat Commun. 2020 Apr 7;11(1):1729. doi: 10.1038/s41467-020-15537-w. Nat Commun. 2020. PMID: 32265438 Free PMC article.
-
Leptin receptor neurons in the dorsomedial hypothalamus require distinct neuronal subsets for thermogenesis and weight loss.Metabolism. 2025 Feb;163:156100. doi: 10.1016/j.metabol.2024.156100. Epub 2024 Dec 12. Metabolism. 2025. PMID: 39672257
-
Dorsomedial hypothalamic NPY modulation of adiposity and thermogenesis.Physiol Behav. 2013 Sep 10;121:56-60. doi: 10.1016/j.physbeh.2013.03.022. Epub 2013 Apr 3. Physiol Behav. 2013. PMID: 23562863 Free PMC article. Review.
-
The TRH neuron: a hypothalamic integrator of energy metabolism.Prog Brain Res. 2006;153:209-35. doi: 10.1016/S0079-6123(06)53012-2. Prog Brain Res. 2006. PMID: 16876577 Review.
Cited by
-
Metabolic homeostasis via BDNF and its receptors.Trends Endocrinol Metab. 2021 Jul;32(7):488-499. doi: 10.1016/j.tem.2021.04.005. Epub 2021 May 4. Trends Endocrinol Metab. 2021. PMID: 33958275 Free PMC article. Review.
-
The Pleiotropic Potential of BDNF beyond Neurons: Implication for a Healthy Mind in a Healthy Body.Life (Basel). 2021 Nov 17;11(11):1256. doi: 10.3390/life11111256. Life (Basel). 2021. PMID: 34833132 Free PMC article. Review.
-
Regulation of Satiety by Bdnf-e2-Expressing Neurons through TrkB Activation in Ventromedial Hypothalamus.Biomolecules. 2023 May 11;13(5):822. doi: 10.3390/biom13050822. Biomolecules. 2023. PMID: 37238691 Free PMC article.
-
The Role of BDNF and TrkB in the Central Control of Energy and Glucose Balance: An Update.Biomolecules. 2024 Mar 31;14(4):424. doi: 10.3390/biom14040424. Biomolecules. 2024. PMID: 38672441 Free PMC article. Review.
-
Role of Dorsomedial Hypothalamus GABAergic Neurons in Sleep-Wake States in Response to Changes in Ambient Temperature in Mice.Int J Mol Sci. 2022 Jan 23;23(3):1270. doi: 10.3390/ijms23031270. Int J Mol Sci. 2022. PMID: 35163194 Free PMC article.
References
-
- Leibel R. L., Rosenbaum M., Hirsch J., Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 332, 621–628 (1995). - PubMed
-
- Lotfi K., Palmer K., Apovian C. M., Case Study: Weight loss in a patient with type 2 diabetes: Challenges of diabetes management. Obesity (Silver Spring) 23 (suppl. 1), S11–S12 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

