Synaptotagmin-1-, Munc18-1-, and Munc13-1-dependent liposome fusion with a few neuronal SNAREs
- PMID: 33468652
- PMCID: PMC7848701
- DOI: 10.1073/pnas.2019314118
Synaptotagmin-1-, Munc18-1-, and Munc13-1-dependent liposome fusion with a few neuronal SNAREs
Abstract
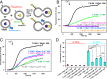
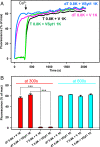
Neurotransmitter release is governed by eight central proteins among other factors: the neuronal SNAREs syntaxin-1, synaptobrevin, and SNAP-25, which form a tight SNARE complex that brings the synaptic vesicle and plasma membranes together; NSF and SNAPs, which disassemble SNARE complexes; Munc18-1 and Munc13-1, which organize SNARE complex assembly; and the Ca2+ sensor synaptotagmin-1. Reconstitution experiments revealed that Munc18-1, Munc13-1, NSF, and α-SNAP can mediate Ca2+-dependent liposome fusion between synaptobrevin liposomes and syntaxin-1-SNAP-25 liposomes, but high fusion efficiency due to uncontrolled SNARE complex assembly did not allow investigation of the role of synaptotagmin-1 on fusion. Here, we show that decreasing the synaptobrevin-to-lipid ratio in the corresponding liposomes to very low levels leads to inefficient fusion and that synaptotagmin-1 strongly stimulates fusion under these conditions. Such stimulation depends on Ca2+ binding to the two C2 domains of synaptotagmin-1. We also show that anchoring SNAP-25 on the syntaxin-1 liposomes dramatically enhances fusion. Moreover, we uncover a synergy between synaptotagmin-1 and membrane anchoring of SNAP-25, which allows efficient Ca2+-dependent fusion between liposomes bearing very low synaptobrevin densities and liposomes containing very low syntaxin-1 densities. Thus, liposome fusion in our assays is achieved with a few SNARE complexes in a manner that requires Munc18-1 and Munc13-1 and that depends on Ca2+ binding to synaptotagmin-1, all of which are fundamental features of neurotransmitter release in neurons.
Keywords: membrane fusion; neurotransmitter release; reconstitution; synaptic vesicle fusion; synaptotagmin-1.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Söllner T., Bennett M. K., Whiteheart S. W., Scheller R. H., Rothman J. E., A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75, 409–418 (1993). - PubMed
-
- Hanson P. I., Roth R., Morisaki H., Jahn R., Heuser J. E., Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell 90, 523–535 (1997). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

